Figure 7:
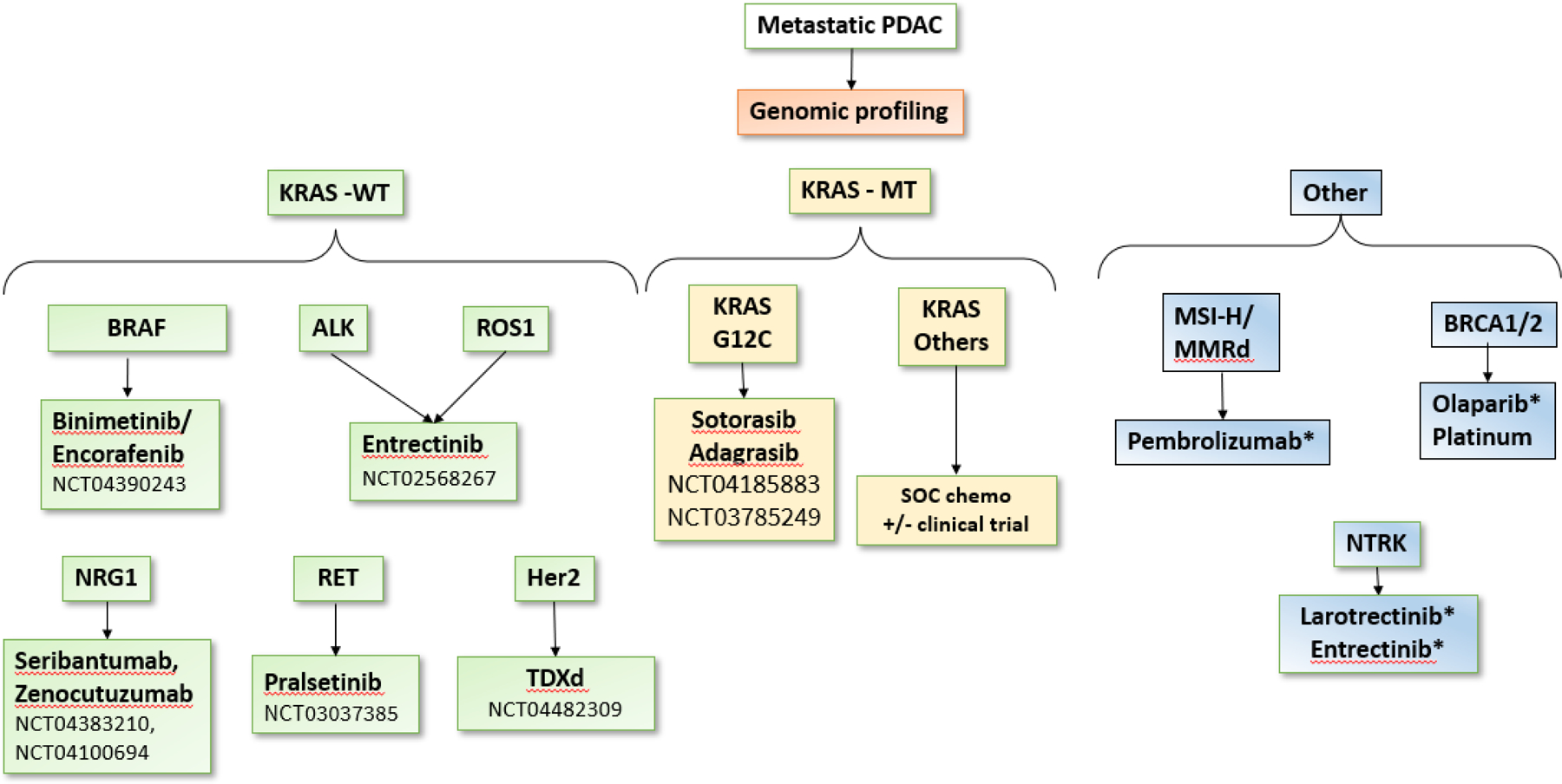
Genomic profiling of advanced pancreatic adenocarcinoma to determine targetable molecular abnormalities. Drugs with an * indicate FDA-approved agents for treatment in pancreatic adenocarcinoma. KRAS WT tumors are enriched with several targetable mutations when compared to KRAS MT tumors. Currently, olaparib approval by the FDA is limited to treating patients with germline BRCA1/2 mutations only. WT: wild type. MT: mutant. PDAC: pancreatic ductal adenocarcinoma. SOC: standard of care.
