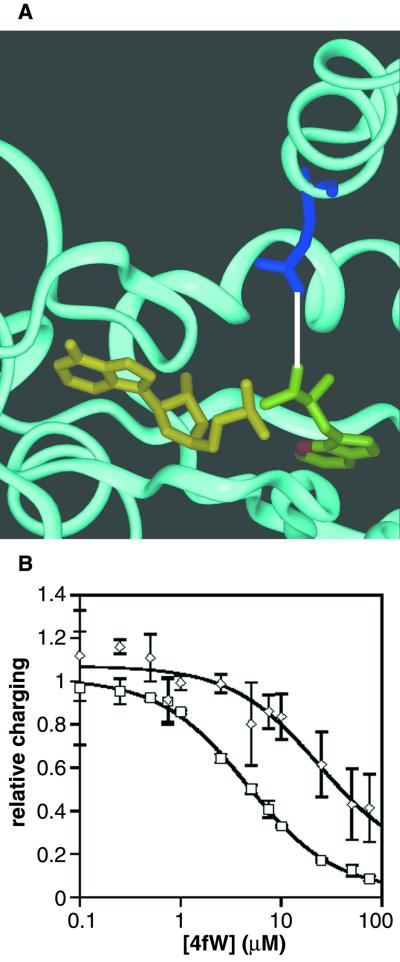
FIG. 9.
Effects of the Q109P mutation on the TrpRS. (A) Ribbon model of tryptophanyl-tRNA synthetase of B. stearothermophilus. Glutamine 107 (corresponding to Q109 of E. coli) (blue) is less than 4 Å (white line) from the Trp-AMP complex (W in green with position 4 in red; AMP in yellow) (10). Q107 (109) is in an α-helical domain near the tRNA docking region. (B) Competition charging assay. Protein was added to a charging assay solution (32) containing constant concentrations of radiolabeled W and competing, unlabeled 4fW. “Relative charging” for wild-type (□) and mutant (◊) proteins is the following ratio: ([3H]W/32P-tRNA)4fW/([3H]W/32P-tRNA)No 4fW. In other words, the charging of tryptophan at a given concentration of 4fW normalized to the charging of tryptophan in the absence of 4fW. Error bars represent the standard deviation of triplicate experiments. Data were fit to the equation used to calculate Vmax,W (see Results and Discussion) by nonlinear regression.