Abstract
Higher cardiac implantable electronic device (CIED) infection incidence has been observed with cardiac resynchronization therapy pacemaker/defibrillator (CRT-P/D) and implantable cardioverter defibrillator (ICD) devices compared to traditional pacemakers with a 1.2% rate reported at 1 year. CIED infection management has high morbidity/mortality. A previous study from this institution demonstrated significantly reduced CIED infection rate when peri/post-operative antibiotics were given for traditional pacemaker procedures. The present study examines CIED infection incidence following peri/post-operative antibiotics during CRT-P/D and ICD procedures. All patients who underwent CRT-P/D and ICD procedures from 1996 to 2015 received IV cephalexin/clindamycin pre- and 8-hours post-procedure followed by 5 days of oral therapy. There were 427 procedures (CRT-P = 146 (34.2%); CRT-D = 142 (33.3%); ICD = 139 (32.6%)). Mean age at time of procedure was 61.6 years. Mean follow-up duration was 4.26 years. CIED infection occurred in 6 patients (ICD = 4, CRT-P = 1, CRT-D = 1), amounting to a rate of 4.96/1000 device-years in total. Times to CIED infection from procedure were: 1.7, 3.5, 6.7, 7.3, 7.9 and 9.2 years. Five out of 6 infections occurred in patients with repeat procedures. This study demonstrates that administration of peri- followed by post-operative antibiotics during CRT-P/D and ICD procedures is associated with a very low rate of CIED infection. This rate of 4.96 infections per 1000 device-years compares favorably to contemporary rates of 8.9 infections per 1000 device-years. Most CIED infections occur late and well-beyond the 1-year follow-up of the Prevention of Arrhythmia Device Infection Trial, the largest trial on this question. This approach should be considered pending a definitive trial
Keywords: antibiotics, cardiac implantable electronic device infection, defibrillators, pacemakers
1. Introduction
Cardiovascular implantable electronic devices (CIED) are now ubiquitous in modern cardiology. Since the initial implantations of CIEDs in the 1960s, these devices have become significantly more reliable with each advancing decade along with advances in technology, implantation technique, as well as device design with expanded indications. However, despite these advances, there remains significant morbidity and mortality associated with CIED infections which are serious complications that may arise following device implants and procedures. Management of these cases involves invasive procedures such as reentering the surgical site, device explantation, lead extraction and surgical debridement which, along with other required treatments, cause significant morbidity and mortality to the patients. A meta-analysis found an all-cause mortality rate of up to 35% following CIED infection, based on 19 studies involving at least 100 patients over a maximum follow-up period of 5.5 years.[1] Endocarditis is associated with an even higher mortality rate than general pocket infections.[1] Known risk factors for CIED infection include device complexity, higher number of prior procedures and absence of antimicrobial prophylaxis.[2] Infection management also has significant hospitalization costs and resource utilization.[3,4] A retrospective study on Medicare beneficiaries post-infection found that the infection-related hospitalized patients accumulated high mean facility-based service costs (mean = $77,397, standard deviation = $79,130).[3,4]
With the advent of new technologies and expanded indications, there has been a significant growth in the use of advanced CIEDs over the last ten years. Unlike the traditional pacemaker, implantable cardioverter defibrillator (ICD) devices are more complex devices that serve an especially high-risk patient population. Cardiac resynchronization therapy pacemakers (CRT-P) and cardiac resynchronization therapy defibrillators (CRT-D) often require 3 leads and have added surgical complexity. Though these advanced CIEDs have more potentially mortality and morbidity saving functions than traditional pacemakers, this comes at the cost of a higher risk of infection. Higher CIED infection rates are observed in CRT-P/D and ICD devices compared to traditional pacemakers. Studies identify a 1.2% infection rate reported at 1 year and an overall rate of 8.9 per 1000 device-years for CRT-P/D and ICD devices.[5] This compares to an infection rate of 4.82 per 1000 device-years reported in Denmark for traditional pacemakers.[6] When comparing CRT to ICD devices, a study out of Italy found CRT-D infection rates as high as 10.0 per 1000 patient-years[7] while an international study of ICD infections yielded a rate of 3.1 per 1000 patient-years.[8]
When comparing first procedure versus repeat procedures, a meta-analysis of CIED infection incidences reported a cumulatively 2 to 5-fold higher incidence for repeat procedure patients when compared to those with first procedures.[3] A prospective study by Johansen et al found an infection rate of 4.82 per 1000 pacemaker-years for first-procedure patients and a rate of 12.12 per 1000 pacemaker-years for repeat procedures.[4]
There are conflicting findings regarding the benefits of antibiotic and antimicrobial prophylaxis during device procedures.[9,10] A previous study from our institution demonstrated a reduced device infection rate from 3.6% (no antibiotics) to 2.9% (perioperative antibiotics only) to 0.4% (peri- plus postoperative antibiotics) when an extended peri- and post-operative antibiotic course of 5 days was given during anti-bradyarrhythmia device procedures.[11] We hypothesize that given that CIED infection rates are higher in CRT-P/D and ICD devices, the use of peri- and post-operative antibiotics may be useful in reducing the CIED infection rate in these complex devices.
2. Materials and methods
2.1. Patient selection
The Grey Nuns Hospital is 1 of 5 acute care hospitals and 1 of 3 pacemaker implanting hospitals in Edmonton, Canada and serves as a referral center for rural hospitals throughout Alberta, Northern British Columbia, the Northwest Territories, and Nunavut. All patients who underwent CRT-P/D and ICD implants and procedures at the Grey Nuns Hospital during the period of 1996 to 2015 were included in this study. Each patient met the standard American College of Cardiology/American Heart Association guidelines for implantation of either an ICD or CRT-P/D device.[12]
The inclusion criteria were:
CRT-P implantations
CRT-P repeat procedures (e.g., pulse generator replacements, lead revisions, hematoma re-explorations)
CRT-D implantations
CRT-D repeat procedures (e.g., pulse generator replacements, lead revisions, hematoma re-explorations)
ICD implantations
ICD repeat procedures (e.g., pulse generator replacements, lead revisions, hematoma re-explorations)
The exclusion criteria were:
Children age <18 years old
Traditional pacemaker implantations
2.2. Surgical procedure
All CRT-P/D and ICD procedures at our site are completed by 1 of 2 electrophysiology certified cardiologists along with a pacemaker nurse and radiation technologist. All procedures are performed in a special pacemaker surgical suite with sterile technique and appropriate local preparation and hand washing. All patients provided written consent for the procedure. All patients received local anesthesia and some patients received midazolam and fentanyl intravenously for sedation and analgesia. Leads were implanted under fluoroscopic guidance. Passive or active leads were chosen at the discretion of the implanter. Devices were interrogated and programmed in the pacemaker suite. Most implantations were done as day procedures.
2.3. Antibiotic therapy
All patients received 2 doses of 1 g intravenous cefazolin. The first dose was administered 30 minutes before skin incision whereas the second dose was administered postoperatively at 8 hours. This was followed by a 4-day course of oral cephalexin 500 mg 4 times/day for a total of 5 days of antibiotics. For patients with a beta-lactam allergy, 2 doses of 600 mg of intravenous clindamycin was used followed by a 4-day course of oral clindamycin 300 mg 3 times/day. These antibiotics were chosen as they are active against contaminating skin flora and are in keeping with most surgical operating theater practices. The duration of therapy was arbitrary with the thought being that a shorter course could be used compared to infectious disease guidelines for skin and soft tissue infections given that this was a prophylactic course. All orders were written on preprinted order sheets in order to ensure consistency between patients.
2.4. Repeat procedures
Some patients had repeat procedures such as pulse generator replacements, lead revisions, hematoma re-explorations. Peri- and post-operative antibiotics were given to all patients undergoing repeat procedures. CIED infection was always ascribed to the last procedure that the patient had prior to the infection.
2.5. Follow-up
All patients had follow-up visits scheduled for 1- and 6-weeks post-procedure. Thereafter, patients were followed every 3–6 months for the first year and every 6 months thereafter. All other visits were considered unscheduled if, for example, a patient presented with symptoms suggestive of a CIED infection. At each follow-up visit, the pacemaker site was checked, lead impedances were measured and pacemakers were interrogated.
2.6. Data collection
All prospectively collected data was retrieved from Web Access Management and PaceArt databases and recorded on customized hardcopy templates. Data collected included patient gender, age at device implant, device type, procedure type, prior implants, antibiotics used, patient deaths, CIED infection, CIED infection type, CIED infection symptoms, post-infection procedures and infection cultures. The template data was then transferred to a database within the Statistical Package for Social Sciences data management system version 21 (SPSS, International Business Machines Corporation, Armonk, NY).
2.7. Statistical analysis
Total follow-up duration was calculated for the entire cohort as well as the different device types by totaling the follow-up time in years for all patients for the entire cohort as well as for each different device type. Continuous variables were analyzed with a Student t test, whereas cross-tabulation analysis was conducted on discrete variables using a χ2 test. CIED infection rate was calculated as the number of CIED infections per 1000 device-years by dividing the number of CIED infections by the total duration of follow-up in years for all patients and then by multiplying by 1000 (as is commonly used to compare device infection rates in the literature).
CIED infections per 1000 device-years = [(number of CIED infections)/(total follow-up duration of all patients in years)] × 1000.
2.8. Outcomes
The primary outcome was CIED infections (per 1000 device-years). Secondary outcomes included time to CIED infection, endocarditis rate, and pocket infection rate. Subgroup analyses were performed for the subgroups of ICD, CRT-P, and CRT-D as well as for first versus repeat procedures. With regards to the definition of CIED infection, all local subcutaneous pocket infections, all pacemaker erosions, and all CIED associated infective endocarditis were included. This all-encompassing definition was used as many believe that pacemaker erosions occur due to local low grade infection which leads to overlying tissue necrosis followed by erosion. If a patient underwent multiple procedures, the last procedure prior to CIED infection was considered the procedure resulting in infection.
2.9. Ethics
The present study received approval from the Health Research Ethics Board – Health Panel at the University of Alberta, Edmonton, Canada (Pro00083745).
3. Results
Between the years of 1996 and 2015, 427 CRT-P, CRT-D and ICD device procedures (implants and repeat procedures) were performed at our site. These procedures were performed on 284 separate patients. The mean age at time of first implantation was 61.6 years. There were 209 males (73.6%) and 75 females (26.4%) in our study population. There were 146 (34.2%) CRT-P procedures, 142 (33.3%) CRT-D procedures, and 139 (32.6%) ICD procedures. Patients were followed up in clinic for a mean duration of 4.3 ± 0.2 (standard error of the mean) years.
CIED infection occurred after 6 procedures (1.4% of total). Based on a total follow-up duration of 1210 device years, this amounts to a rate of 4.96 infections per 1000 device-years in total (Table 1). Times to CIED infection from last procedure were 1.7, 3.5, 6.7, 7.3, 7.9 and 9.2 years. 5 infections were pocket infections, and 1 was endocarditis. The patient with endocarditis grew Staphylococcus aureus in blood. Two patients had Coagulase negative Staphylococcus in their pocket cultures, 1 patient had Propionibacterium acnes in the pocket culture, and the other 2 did not grow anything from the pocket. Of the 6 total infections, 1 (0.7%) occurred after CRT-P procedures, 1 occurred after CRT-D procedures (0.7%), and 4 (2.9%) occurred after ICD procedures. This amounts to an infection rate of 2.08 infections per 1000 device-years for CRT-P procedures (total patient follow-up duration = 480.23 years), 3.36 infections per 1000 device-years for the CRT-D procedures (total patient follow-up duration = 297.52 years), and 9.34 infections per 1000 device-years for the ICD procedures (total patient follow-up duration = 428.43 years).
Table 1.
Cardiac implantable electronic device infection rate by type of pacemaker and type of procedure.
| Procedure/pacemaker type | Infection rate (number per 1000 device-years) |
|---|---|
| All complex pacemakers (CRT-P, CRT-D, ICD) | 4.96 |
| First procedures, all types (CRT-P, CRT-D, ICD) | 1.91 |
| Repeat procedures, all types (CRT-P, CRT-D, ICD) | 7.32 |
| CRT-P procedures, all | 2.08 |
| CRT-D procedures, all | 3.36 |
| ICD procedures, all | 9.34 |
CRT-D = cardiac resynchronization therapy defibrillator; CRT-P = cardiac resynchronization therapy—pacemaker; ICD = implantable cardioverter defibrillator.
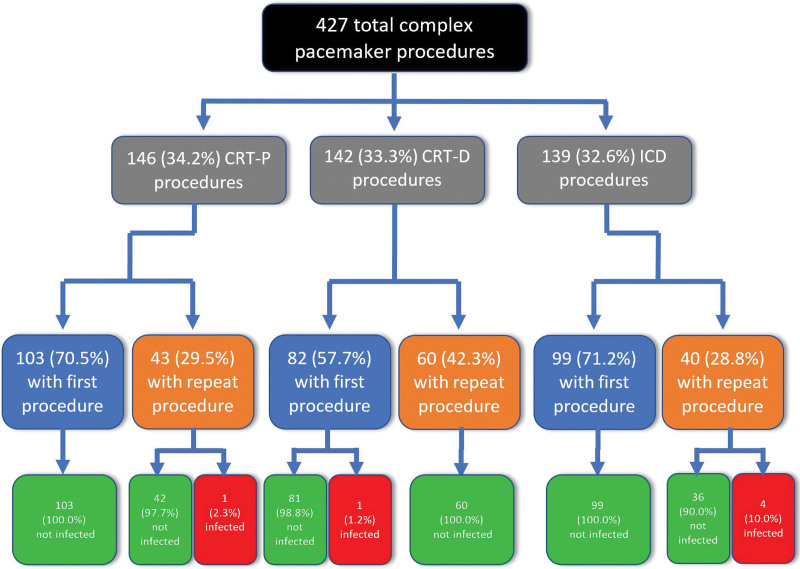
Of the 284 patients, 182 (64.1%) had only the initial new implantation. 68 (23.9%) had 1 repeat procedure, 29 (10.2%) had 2 repeat procedures, 3 (1.1%) had 3 repeat procedures, and 2 (0.7%) had 4 repeat procedures for a total of 143 repeat procedures out of 427 total procedures (Fig. 1). Out of 103 patients with CRT-P devices, 29 (28.2%) underwent at least 1 repeat procedure. Out of 82 patients with CRT-D devices, 41 (50.5%) underwent at least 1 repeat procedure. Out of 99 patients with ICD devices, 32 (32.3%) underwent at least 1 repeat procedure. In the CRT-P patients, there were a total of 43 repeat procedures: 19 (44.2%) were pulse generator replacement (PGR) procedures, 10 (23.3%) were lead procedures, 9 (20.9%) were a combination of PGR and lead procedures and 5 (11.6%) were other procedures. In the CRT-D patients, there were a total of 60 repeat procedures: 21 (35.0%) were PGR procedures, 33 (56.0%) were lead procedures, 4 (6.7%) were a combination of PGR and lead procedures, and 2 (3.3%) were other procedures. Of the ICD patients, there were a total of 40 repeat procedures: 17 (42.5%) were PGR procedures, 15 (37.5%) were lead procedures, 2 (5.0%) were a combination of PGR and lead procedures and 6 (15.0%) were other procedures. When comparing repeat procedures to first procedures, the infection rate was 7.32 infections per 1000 device-years for repeat procedures and 1.91 infections per 1000 device-years for first procedures. All pocket infections occurred in patients with repeat procedures. Figure 2 gives a breakdown of CIED infection based on both type of device and first versus repeat procedure.
Figure 1.
Patient cohort of new implants compared to those patients that had 1 or more repeat subsequent procedures.
Figure 2.
Flow diagram of CRT-P, CRT-D and ICD patient characteristics. CRT-D = cardiac resynchronization therapy defibrillator; CRT-P = cardiac resynchronization therapy—pacemaker; ICD = implantable cardioverter defibrillator.
4. Discussion
The present study looks at a cohort of patients with complex pacemaker procedures (CRT-P, CRT-D, and ICD implants) who received peri- plus post-operative antibiotics during CIED implantation and repeat procedures. CIED infection remains an important problem in this highly comorbid group and our study has several important findings with regards to the efficacy of peri- and post-operative antibiotics in reducing CIED infection rate, the time to CIED infection, and the risk in repeat procedures in the different types of complex pacemakers.
A strategy of peri- and post-operative antibiotics appears to favorably reduce the CIED infection rate compared to historical controls. Our findings suggest that applying peri- plus post-operative antibiotics during CIED procedures reduces the rate of infection post-procedure. Our infection rate of 4.96 infections per 1000 device-years in this study, compares favorably to other contemporary studies such as a reported rate of 8.9 infections per 1000 device-years by Uslan et al in complex devices.[5] This is also in keeping with our previous findings in traditional pacemakers showing a reduction in CIED infections with peri- and post-operative antibiotic use.[11]
In general, CIED infection is a late complication. We found that all CIED infections in this study occurred well beyond the 1-year mark of the last procedure. This is similar to findings from other trials that have suggested that CIED infection is a late rather than early complication. In Bluhm et al, CIED infection onset was up to 18 months after the last procedure, and in Sohal et al, the median time to infection was 415 days from last procedure.[13,14] A large cluster randomized crossover trial on peri-operative antibiotics (the Prevention of Arrhythmia Device Infection Trial – PADIT trial) was published in 2018 and found no reduction in CIED infection with peri-operative antibiotics and is the biggest trial on this question.[15] However, the major criticism of this trial was that it only followed patients for 1-year and as a result many CIED infections were likely missed. The authors themselves comment on a surprisingly low CIED infection rate of approximately 1% in both arms which would again be in keeping with too short a follow-up to see the incidence of CIED infection and therefore the value of peri- and post-operative antibiotics.
The ICD group appears to have a particularly high rate of CIED infection. Unfortunately, we do not have enough data to see if this is due to the ICD procedure itself or rather the type of patients that ICDs are inserted into who may be more comorbid. CRT-P devices specifically are likely used in less comorbid patients requiring only pacing.
Our research also aligns with previous findings that repeat procedures greatly increase the risk of CIED infection. 5 out of the 6 infected patients were those with repeat procedures (yielding an infection rate of 7.32 infections per 1000 device-years). This suggests that revision procedures increase risk of infection, and this finding corresponds with both the meta-analysis by Sandoe et al and the study by Johansen et al which found higher infection rates for repeat-procedure patients.[3,4]
4.1. Limitations
This study is limited by its observational nature and its basis on a single regimen of antibiotic therapy. Conducting a randomized controlled trial with sufficient follow-up time to catch late CIED infection would provide further insight into the effectiveness of peri- and post-operative antibiotic regimens. The long term follow-up results from the PADIT trial would also be interesting to potentially help answer this question.
5. Conclusions
This study demonstrates that peri-operative antibiotic administration followed by an extended 5-day post-operative antibiotic course during CRT-P/D and ICD procedures is associated with a low CIED infection rate. There were no CIED infections in our cohort within the first year, which compares favorably to 1.2% in contemporary cohorts. Most CIED infections occur late, well beyond the 1-year follow-up period of the largest randomized controlled trial (PADIT) on this question. Our rate of 4.96 infections per 1000 device-years compares favorably to contemporary reported rates of 8.9 infections per 1000 device-years in patients with CRT-P/D and ICDs. This approach should be considered pending a definitive trial in view of the increasing incidence of CIED.
Author contributions
Conceptualization: Janek Manoj Senaratne, Jessica Wijesundera, Usha Chhetri, Diane Beaudette, Andrea Sander, Mike Hanninen, Sajad Gulamhusein, Mano Senaratne.
Data curation: Janek Manoj Senaratne, Jessica Wijesundera, Diane Beaudette, Andrea Sander, Mike Hanninen, Sajad Gulamhusein, Mano Senaratne.
Formal analysis: Janek Manoj Senaratne, Jessica Wijesundera, Mano Senaratne.
Funding acquisition: Janek Manoj Senaratne, Mano Senaratne.
Investigation: Janek Manoj Senaratne, Jessica Wijesundera, Usha Chhetri, Diane Beaudette, Sajad Gulamhusein, Mano Senaratne.
Methodology: Janek Manoj Senaratne, Mano Senaratne.
Project administration: Janek Manoj Senaratne, Mano Senaratne.
Resources: Janek Manoj Senaratne, Mano Senaratne.
Software: Janek Manoj Senaratne, Mano Senaratne.
Supervision: Janek Manoj Senaratne, Mano Senaratne.
Validation: Janek Manoj Senaratne, Mano Senaratne.
Visualization: Janek Manoj Senaratne, Mano Senaratne.
Writing – original draft: Janek Manoj Senaratne, Mano Senaratne.
Writing – review & editing: Janek Manoj Senaratne, Mano Senaratne.
Disclosure statement
There are no conflicts of interest to disclose.
Abbreviations:
- CIED =
- Cardiovascular implantable electronic device
- CRT-D =
- Cardiac resynchronization therapy defibrillator
- CRT-P =
- Cardiac resynchronization therapy pacemaker
- ICD =
- Implantable cardioverter defibrillator
- PADIT =
- Prevention of Arrhythmia Device Infection Trial
- PGR =
- Pulse generator replacement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
The present study received approval from the Health Research Ethics Board – Health Panel at the University of Alberta, Edmonton, Canada (Pro00083745).
How to cite this article: Senaratne JM, Wijesundera J, Chhetri U, Beaudette D, Sander A, Hanninen M, Gulamhusein S, Senaratne M. Reduced incidence of CIED infections with peri- and post-operative antibiotic use in CRT-P/D and ICD procedures. Medicine 2022;101:40(e30944).
Contributor Information
Usha Chhetri, Email: usha.chhetri@covenanthealth.ca.
Diane Beaudette, Email: diane.beaudette@covenanthealth.ca.
Mike Hanninen, Email: hanninen@ualberta.ca.
Sajad Gulamhusein, Email: sajad@ualberta.ca.
Mano Senaratne, Email: manosenaratne@shaw.ca.
References
- [1].Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2014;70:325–59. [DOI] [PubMed] [Google Scholar]
- [2].Johansen JB, Jorgensen OD, Moller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Greenspon AJ, Eby EL, Petrilla AA, et al. Treatment patterns, costs, and mortality among medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018;41:495–503. [DOI] [PubMed] [Google Scholar]
- [4].Sohail MR, Henrikson CA, Braid-Forbes MJ, et al. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171:1821–8. [DOI] [PubMed] [Google Scholar]
- [5].Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–75. [DOI] [PubMed] [Google Scholar]
- [6].Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Landolina M, Gasparini M, Lunati M, et al. Long-term complications related to biventricular defibrillator implantation: rate of surgical revisions and impact on survival: insights from the Italian clinical service database. Circulation. 20112011;123:2526. [DOI] [PubMed] [Google Scholar]
- [8].Gold MR, Peters RW, Johnson JW, et al. Complications associated with pectoral cardioverter-defibrillator implantation: comparison of subcutaneous and submuscular approaches. Worldwide Jewel Investigtors. J Am Coll Cardiol. 1996;28:1278–82. [DOI] [PubMed] [Google Scholar]
- [9].Lee W, Huang T, Lin L, et al. Efficacy of postoperative prophylactic antibiotics in reducing permanent pacemaker infections. Clin Cardiol. 2017;40:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Darouiche R, Mosier M, Voigt J. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. Pacing Clin Electrophysiol. 2012;35:1348–60. [DOI] [PubMed] [Google Scholar]
- [11].Senaratne J, Jayasuriya A, Irwin M, et al. A 19-year study on pacemaker-related infections: a claim for using postoperative antibiotics. Pacing Clin Electrophysiol. 2014;37:947–54. [DOI] [PubMed] [Google Scholar]
- [12].Gregoratos G, Cheitlin MD, Conill A, et al. ACC/AHA guidelines for implantation of cardiac pacemakers and antiarrhythmia devices: executive summary – a report of the American College of Cardiology/American Health Association task force on practice guidelines (committee on pacemaker implantation). Circulation. 1998;97:1325. [DOI] [PubMed] [Google Scholar]
- [13].Bluhm GL. Pacemaker infections. A 2-year follow-up of antibiotic prophylaxis. Scand J Thorac Cardiovasc Surg. 1985;19:231–5. [DOI] [PubMed] [Google Scholar]
- [14].Sohail MR, Uslan DZ, Khan AK, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–9. [DOI] [PubMed] [Google Scholar]
- [15].Krahn AD, Longtin Y, Philippon F, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018;72:3098–109. [DOI] [PubMed] [Google Scholar]