Summary
Coeliac disease is a lifelong immune‐mediated enteropathy with systemic features associated with increased morbidity and modestly increased mortality. Treatment with a strict gluten‐free diet improves symptoms and mucosal damage but is not curative and low‐level gluten intake is common despite strict attempts at adherence. Regular follow‐up after diagnosis is considered best‐practice however this is executed poorly in the community with the problem compounded by the paucity of data informing optimal approaches. The aim of dietary treatment is to resolve symptoms, reduce complication risk and improve quality of life. It follows that the goals of monitoring are to assess dietary adherence, monitor disease activity, assess symptoms and screen for complications. Mucosal disease remission is regarded a key measure of treatment success as healing is associated with positive health outcomes. However, persistent villous atrophy is common, even after many years of a gluten‐free diet. As the clinical significance of asymptomatic enteropathy is uncertain the role for routine follow‐up biopsies remains contentious. Symptomatic non‐responsive coeliac disease is common and with systematic follow‐up a cause is usually found. Effective models of care involving the gastroenterologist, dietitian and primary care doctor will improve the consistency of long‐term management and likely translate into better patient outcomes. Identifying suitable treatment targets linked to long‐term health is an important goal.
1. INTRODUCTION
Coeliac disease is a lifelong immune‐mediated enteropathy associated with important systemic manifestations. 1 , 2 A strict gluten‐free diet (GFD) is the cornerstone of management but does not resolve the underlying immune cause of coeliac disease. 3 The GFD imparts a substantial treatment burden, 4 and many patients fail to achieve complete symptom or mucosal remission. As coeliac disease is associated with increased morbidity and modestly increased mortality, periodic medical follow‐up is considered a crucial component of patient care 5 , 6 , 7 , 8 but this is compromised by the paucity of evidence to inform best‐practice approaches. 9 , 10 As a result, real‐world follow‐up is often inconsistent or absent altogether. 11 , 12 , 13 For many chronic illnesses, such as type 1 diabetes, the importance of maintaining long‐term follow‐up to assess disease status, treatment efficacy and monitor for complications is well established in the medical community, however, for coeliac disease, this is typically not the case, even though the monitoring goals are the same. 5 , 6 , 7 , 8 , 14 For patients, the key goals of treatment are to resolve symptoms, reduce the risk of complications and achieve optimal quality of life, and for clinicians, disease remission also encompasses healing of the enteropathy. Effective models of care that leverage local medical and allied health expertise, ideally involving a gastroenterologist, dietitian and primary care provider, will support these goals but will require flexibility to ensure they can be practically implemented across a range of patient populations. Appropriate follow‐up in developing nations where there are challenges with medical infrastructure and resources is an unmet need.
2. MUCOSAL HEALING
2.1. Morbidity and mortality in coeliac disease
Mucosal healing is a key goal of treatment (Figure 1). While this is well accepted, the evidence base to support mucosal remission in improving outcomes in coeliac disease is relatively modest, as the large, prospective studies needed to demonstrate a clear benefit are challenging to perform. Observational studies lend support to this goal, but retrospective studies can be limited by selection bias. For example, follow‐up biopsies are often reserved for clinically unwell patients with pathology, so the significance of asymptomatic enteropathy at a population level remains poorly defined.
FIGURE 1.
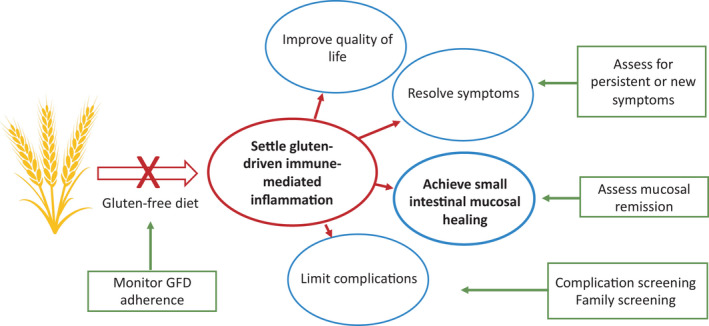
Goals of treatment and follow‐up. Reducing gluten‐induced immune inflammation is the key goal of treatment and currently, this is achieved with a gluten‐free diet (GFD). Successful treatment will lead to mucosal healing, resolve symptoms, reduce the risk of complications and improve quality of life. A strict GFD is not a goal of treatment but the current means to achieve treatment outcomes, and eventually may be superseded by more effective approaches. The key components of medical follow‐up aim to monitor the outcomes highlighted in the green boxes
Persistent villous atrophy and inflammation are associated with greater morbidity, such as the increased risk of hip fractures 15 and lymphoproliferative malignancy 16 and mucosal healing improves outcomes. For example, compared with the general population, coeliac patients with persistent villous atrophy have a 3.78‐fold (CI 2.71–5.12) increase in lymphoproliferative malignancy, while those with mucosal healing have a 1.5‐fold (CI 0.77–2.62) increase. To put this risk in perspective, the absolute risk of lymphoproliferative malignancy in coeliac disease is low, approximately 70 per 100,000 person‐years. 17 Coeliac disease is associated with modestly increased mortality 18 , 19 , 20 but an association between persistent villous atrophy and mortality has not been shown. 21 Likewise, the effect of a GFD on mortality is unclear, as the data are limited to retrospective studies at risk of bias.
A GFD clearly benefits some complications, such as reduced bone mineral density or abnormal transaminases. However, the degree of reversibility depends on the complication and its severity; for example, the benefit of a GFD in established osteoporosis is more modest than in osteopenia. 9 The benefits of a GFD in reducing gluten‐induced inflammation and end‐organ effects translate into a significant restoration of quality of life. 22 , 23 , 24 , 25
2.2. What is the goal of healing?
The degree of healing that constitutes sufficient mucosal disease remission is uncertain. It remains unclear if normal architecture with raised intraepithelial lymphocytes (Marsh 1) is similarly acceptable to complete mucosal normalisation as Marsh 1 changes have been linked to a modest mortality increase in coeliac disease. 19 The significance of enteropathy confined to the duodenal bulb is unknown. 26 We must sensitively communicate the goals of treatment to our patients, as those conscientiously avoiding gluten are understandably upset and frustrated by the finding of persistent enteropathy, and hypervigilance and fear of gluten exposure are associated with greater anxiety and depression. 27
Coeliac patients show highly variable mucosal healing rates on a GFD in follow‐up studies ranging from 6 months to 10 years. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Rates of healing after 2 years are between 12% and 79% with variability likely resulting from retrospective studies, how mucosal recovery was defined and histology reported, and variations in GFD duration, GFD adherence and patient age. 41 Mucosal healing in children is also variable but tends to be faster and more complete than in adults (generally >80% at 2 years). 39 , 42 , 43 , 44 A prospective study of Spanish adults showed villous atrophy affected 40/76 (53%) after 2 years despite rigorous GFD education and monitoring. 37 In 93 US and Australasian adults with coeliac disease on a strict GFD for a median of 6 years, mucosal disease was common (58% Marsh 3a/b and 35% Marsh 2), with minor changes in 6% (Marsh 1) and normal histology (Marsh 0) in only 1%; 90% of those with villous atrophy were transglutaminase antibody negative. 33 These striking findings were based on quantitative morphometry, the gold‐standard histological reporting approach for clinical trials favoured for its excellent accuracy and reliability. 45 , 46 Collectively, the data support the notion that persistent enteropathy affects most adult coeliac patients on a long‐term GFD, even those with strict adherence and negative coeliac serology.
While any degree of villous atrophy and inflammation is intuitively undesirable, the prognostic significance of persistent enteropathy is poorly understood, particularly in the asymptomatic individual. Patients with suboptimal GFD adherence lost to care will not be captured in follow‐up studies. While asymptomatic enteropathy has been associated with an increased risk of osteoporosis, refractory coeliac disease and malignancy, 47 the study was small (n = 13) and potentially affected by referral bias. Nevertheless, the finding may support more intensive dietary management and closer monitoring for complications, such as reduced bone density.
Is mucosal healing a realistic goal in all patients with a GFD alone? Although a 99% reduction of gluten intake is achievable with a GFD (based on daily average estimates of 15 g dietary gluten intake 48 and 150 mg inadvertent exposure), 49 it seems likely that the GFD for many, if not most, patients is insufficient to induce complete symptomatic and mucosal remission. We need to understand the significance of persistent enteropathy to inform treatment goals and focus efforts on developing better therapeutics more effective than the GFD alone. 50
2.3. Role for follow‐up biopsies
There is broad agreement that follow‐up biopsies are warranted in patients with persistent symptoms on a strict GFD. However, the role of routine follow‐up biopsies, especially for patients with negative coeliac serology, replete nutritional markers and stable bone density, remains contentious. Surrogate markers of intestinal healing, such as normalisation of coeliac serology, are frequently used in the clinic and are simple, minimally invasive and preferred by patients, but they have poor predictive value for mucosal healing. 9 , 26 , 35 , 51 Non‐invasive molecular markers of mucosal healing have been assessed, for example intestinal fatty acid‐binding protein (I‐FABP), but lack sufficient utility to inform clinical practice. 52 Small intestinal histology is the only approach that can accurately assess mucosal activity, but is highly dependent on sufficient duodenal sampling (multiple biopsies across several parts of the duodenum), well‐oriented biopsies and standardised histopathological interpretation 45 ; however, there is scepticism that this can be consistently achieved in clinical practice. 33 , 53
Follow‐up biopsies can inform clinical decision‐making. In symptomatic patients, or those with nutrient deficiencies or worsening bone mineral density, histological reassessment can help determine if these issues can be attributed to persistent coeliac disease activity. This supports intensification of dietary management, including evaluation of knowledge and practices, and possible consideration for more stringent dietary approaches such as the gluten contamination elimination diet 54 or adjunctive medication. If refractory coeliac disease is diagnosed, specialist management can be initiated. Reassessment allows other gastrointestinal issues, for example gastritis to be identified and managed. If the intestinal mucosa is healed, other causes for the persistent issues can be examined (discussed below). In asymptomatic patients, or those who were coeliac serology‐negative at diagnosis, histological reassessment is the only informative readout of disease activity. Importantly, histological assessment involves our patients with their ongoing care, and the findings can often be used to empower and support them in maintaining dietary adherence.
An argument against routine follow‐up biopsies has been the lack of treatment approaches beyond the GFD if enteropathy is found. This situation is changing as the unmet need for effective therapies in persistently active coeliac disease is increasingly acknowledged by industry and novel therapies for this indication are in the pipeline. 50 Open‐capsule budesonide, widely employed in refractory coeliac disease, 55 has shown promise in a retrospective study of non‐responsive coeliac in improving symptoms in 57% and villous damage in 46%. 56 The availability of pharmacological options that induce mucosal remission will strengthen the argument for histological reassessment.
2.4. Timing of endoscopy
The optimal timing for endoscopic follow‐up, if undertaken at all, is unclear. In adults, repeat endoscopy after 2 years on a GFD is often recommended to confirm mucosal healing if the patient has clinically stabilised and coeliac serology has normalised. If not done routinely, then it should be strongly considered in patients at higher risk of incomplete recovery, such as those with severe initial mucosal damage 57 or presentation or older age at diagnosis, for example 40 and above. 5 , 39 Earlier endoscopy is indicated in the clinically non‐responsive patient. For patients who have achieved mucosal remission on a GFD, there are no current data to support the need for ongoing endoscopies if the patient remains clinically well.
3. MONITORING THE GFD
A strict and lifelong GFD is challenging and imparts a substantial treatment burden. 4 Strict dietary adherence is variable, ranging between 42% and 91%. 58 In practice, strict avoidance of gluten is aspirational rather than completely achievable, with recent studies highlighting a high rate of gluten exposure even in patients with apparently strict adherence. 37 , 59 , 60 , 61 An important contributor is gluten cross‐contamination, which affects 2.1%–37% of labelled and non‐labelled gluten‐free food products. 62 Periodic follow‐up to monitor GFD adherence based on history and coeliac serology is recommended. 5 , 6 , 7 , 8 However, while a history of dietary non‐adherence or positive coeliac serology is strongly associated with intestinal damage, a history of dietary adherence and negative serology poorly predict healed mucosa. 30 The following approaches may be used to help build a picture of dietary adherence but biopsies are the fallback approach when mucosal healing needs to be assessed accurately.
3.1. Dietitian review
Dietitian‐led evaluation of the GFD is highly valuable for identifying gaps in knowledge or practices leading to inadvertent gluten ingestion. While traditionally considered closest to a gold standard, it is time consuming and limited by interobserver variability and lack of standardisation. Even when patients have good knowledge, practices and motivation to follow a strict GFD, their attempts may be thwarted by inadvertent gluten cross‐contamination, a common problem. 63 , 64 , 65 As a major cause of persistent symptoms in coeliac disease is irritable bowel syndrome, 66 , 67 a dietitian is ideally placed to initiate therapy with a low fermentable carbohydrate (FODMAP) diet 68 and advise on the practical challenges of combining this treatment with a GFD.
3.2. Dietary adherence questionnaires
GFD adherence questionnaires are simple, rapid to administer and provide a quantitative readout of dietary adherence. However, both the 4‐item 69 and the 7‐item Celiac Dietary Adherence Test 70 questionnaires have poor sensitivity in detecting villous atrophy, of 25%–33% 43 , 71 and 55%, 26 respectively. While useful for clinical studies, the practical application of dietary questionnaires in the clinic appears limited.
3.3. Symptom assessment
Improvement of coeliac‐related symptoms on a GFD is an encouraging marker of treatment response and can occur within days to weeks. 72 Poor clinical response coupled with poor GFD adherence is linked to a high risk of persistent villous atrophy (46%; 95% CI 25–68). 73 However, in isolation, the presence or absence of symptoms on a GFD are poor predictors of mucosal healing 74 and are not informative in patients who have few or no symptoms at diagnosis. Interestingly, some initially asymptomatic patients report subsequent sensitivity to accidental gluten exposure after some time on a GFD. Symptoms of coeliac disease are heterogeneous and can be caused by other issues such as irritable bowel syndrome. 66 , 67 Psychosocial factors may be more important than disease indices such as villous damage in determining gastrointestinal symptoms and health‐related quality of life. 75
3.4. Nutrient assessment
Nutrient deficiencies are common in coeliac disease at diagnosis and during treatment and mostly relate to impaired intestinal absorption. 41 , 76 , 77 , 78 Although poorly predictive of mucosal healing, the presence of nutrient deficiencies such as low iron can be a clinical clue suggestive of active disease.
3.5. Coeliac serology
The circulating levels of antibodies to transglutaminase, endomysium and deamidated gliadin peptides provide an indirect immune readout of disease activity. 79 Coeliac serology generally normalises on a GFD, mostly by 24–36 months. 80 The amount and duration of gluten required to trigger an increase are highly variable. 81 While coeliac serology is frequently used as a surrogate marker of intestinal healing they each have poor sensitivity for detecting villous atrophy (50% for transglutaminase antibodies), with similar levels of performance in paediatric and adult patients. 51 During clinical follow‐up, maintenance of a normal transglutaminase antibody level is often taken to be a reassuring marker of dietary adherence, 82 however, normal values are not predictive of mucosal recovery. 33 , 35 , 83 , 84 The main value of serological monitoring is as a marker of non‐adherence to the diet, as a failure to normalise values or persistently positive values on a long‐term GFD suggests ongoing gluten ingestion. Coeliac serology lacks sensitivity to detect small or infrequent dietary indiscretions. The normalisation of coeliac serology can be used to positively reinforce GFD adherence with our patients, but the focus should be on the trend rather than absolute values.
3.6. Gluten immunogenic peptide assessment
The detection of gluten immunogenic peptides (GIPs) in urine or faeces provides an objective measure of dietary gluten intake, 85 , 86 , 87 but important questions remain regarding how they can be best utilised in the clinic. The GIP ELISA assay detects a major wheat alpha‐gliadin peptide and is highly sensitive, and a less‐studied lateral flow kit allows patients to perform the test at home. Observational studies employing the GIP assay highlight unintended gluten ingestion in treated coeliac disease is common, with higher rates seen with more regular testing over longer intervals. Positive urine or faecal GIP in treated coeliac disease ranges from 25% to 48% (one or two total samples), 86 , 88 , 89 to 69% (twice weekly faecal GIPs performed four times over 2 years) 37 and up to 89% (three‐times per week for 4 weeks). 61 A study coupling assessment of food intake and GIP excretion confirmed the dietary origin for positive GIPs and showed most gluten exposures were asymptomatic and unsuspected. 60 Objective testing of gluten intake is likely to produce confronting results for patients who are strict with their GFD.
GIP testing is superior to dietary questionnaires, coeliac serology and symptom status to detect dietary transgressions, 88 , 90 but the relationship between a positive GIP and villous atrophy is less clear. Positive GIP correlated with villous atrophy in 25 coeliac patients at 2‐year follow‐up, where all those with quantifiable GIP showed incomplete intestinal mucosal recovery and 89% with no villous atrophy had no detectable urine GIP. 89 However, recent studies have failed to show a relationship between positive GIP and villous atrophy. 37 , 59 GIP testing is increasingly being utilised in clinical trials as a tool to confirm the adequacy of the GFD. More studies are needed to understand the relationship between GIP detection and villous atrophy, and if very low levels of GIP are clinically significant and correlate with persistent enteropathy. An understanding of the kinetics of GIP excretion after real‐world gluten exposure is needed to inform the optimal timing and application of these tests in the clinic. Greater availability of GIP testing and clarification of their utility in real‐world settings will support more widespread implementation.
4. MONITORING FOR COMPLICATIONS
Gluten drives the enteropathy of coeliac disease through the activation of gluten‐specific T cells that orchestrate a cascade of pro‐inflammatory events leading to mucosal destruction and systemic cytokine release. 79 , 91 Given this systemic response, coeliac disease is associated with a wide range of complications that can develop at any stage, with some more common after protracted gluten exposure (Table 1). 9 As the development of these complications can be silent or difficult to detect, early recognition through screening tests is important. Periodic follow‐up after diagnosis is recommended 5 , 6 , 7 , 8 , 14 , 92 and a suggested approach is outlined in Figure 2.
TABLE 1.
Clinical associations in coeliac disease. Adapted from Haines et al. 9
| Nutritional deficiencies |
| Iron |
| Folate |
| Vitamin D |
| Vitamin B12 |
| Zinc |
| Calcium |
| Copper |
| Magnesium |
| Autoimmune disease |
| Thyroid disease |
| Type 1 diabetes mellitus |
| Sjogren’s syndrome |
| Systemic lupus erythematosus |
| Alopecia areata |
| Addison’s disease |
| Sarcoidosis |
| Liver disease |
| Hypertransaminasaemia (“coeliac hepatitis”) |
| Metabolic dysfunction‐associated fatty liver disease |
| Autoimmune hepatitis |
| Primary biliary cholangitis |
| Primary sclerosing cholangitis |
| Gastrointestinal |
| Microscopic colitis |
| Small intestinal bacterial overgrowth |
| Pancreatitis |
| Neuropsychiatric disease |
| Neurological abnormalities such as peripheral neuropathy |
| Gluten ataxia |
| Depression |
| Anxiety |
| Reproduction function |
| Sexual dysfunction (males and females) |
| Hypogonadism (males) |
| Menstrual abnormalities |
| Infertility |
| Obstetric problems, e.g. miscarriage, small for gestational age |
| Bone disease |
| Osteopaenia |
| Osteoporosis |
| Fractures |
| Infection |
| Bacterial sepsis, e.g. pneumococcal |
| Tuberculosis |
| Immune deficiency |
| IgA deficiency |
| Cardiac disease |
| Pericarditis |
| Myocarditis |
| Cardiomyopathy |
| Malignancy |
| Lymphoma‐ and enteropathy‐associated T‐cell lymphoma |
| Small bowel adenocarcinoma |
| Oesophageal squamous cell carcinoma |
| Hyposplenism |
| Venous thromboembolism |
| Impaired quality of life |
FIGURE 2.
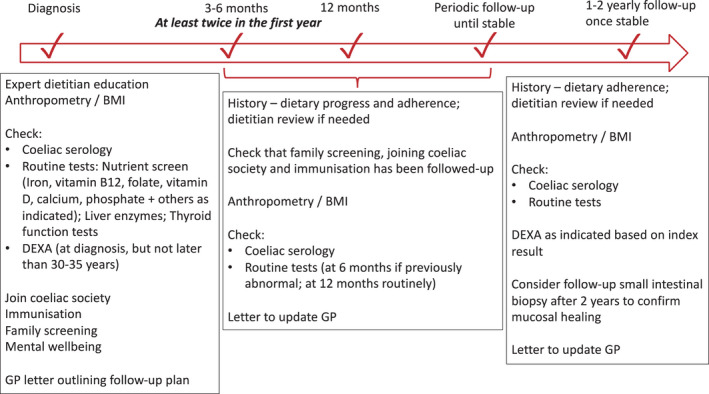
Suggested algorithm for follow‐up. Regular follow‐up is important, especially in the first year. Patients should be reviewed every 3–6 months until well. Once they are symptom free, have replete nutrients and normal serology (and if performed, normal histology), follow‐up can be extended to once every 1–2 years
4.1. Regular assessment
Routine assessment should evaluate GFD adherence, determine nutritional status and monitor for complications based on a combination of history, coeliac serology and other lab‐based tests. A dietitian with expertise in coeliac disease is vital for providing GFD education and reviewing its progress, including the adequacy of GFD knowledge and practices. Dietary review is particularly useful in patients struggling with motivation or when poor adherence is suspected. Dietitians play an important role in promoting healthy eating behaviour, including expanding patient food choices and discouraging unnecessarily restrictive dietary practices related to gluten or other foods. Involving and educating family members is helpful, particularly for young and elderly patients, where the carer may be the person making food choices.
It is recommended that in the first year after diagnosis, monitoring be undertaken every 3–6 months until stable, and 1–2 yearly thereafter. Regular follow‐up in the first year helps reinforce GFD knowledge and provides feedback and encouragement. Professional engagement is important in supporting the patient’s “journey to recovery” and enhances motivation and adherence to the GFD. Patients should be encouraged to join their local coeliac advocacy organisation, which are important sources of information and support, and help promote adherence. 93 This is particularly valuable to initiate early on when the patient is coming to terms with their new diagnosis.
Macro‐ and micronutrient deficiencies are common in coeliac disease at diagnosis and during treatment. 41 , 76 , 77 , 78 Iron deficiency is found in 12–82% 41 and should be treated with an iron infusion when patients are symptomatic or the response to oral supplementation is poor (common before the enteropathy improves). Folate, vitamin B12 and vitamin D should also be measured and any deficiencies corrected. 5 , 6 , 7 , 8 Other reported deficiencies include albumin, zinc, copper, calcium and magnesium and testing should be considered particularly in the coeliac patient with malabsorption or refractory disease. 5 , 76 As the GFD is low in fibre, constipation is common and fibre supplements can be beneficial. Anxiety and depression are common and can impact quality of life and dietary adherence, so psychological input is important. 27 , 94 The pregnant coeliac patient should be closely monitored, as active coeliac disease is associated with poorer foetal outcomes such as low birth weight and miscarriage, possibly through the effect of transglutaminase antibodies which are anti‐angiogenic and can impair placental formation and function. 95
4.2. Autoimmune disease
Autoimmune screening is recommended because of the increased risk (16% in coeliac disease compared to 5% in the general population 96 ) thought to be related primarily to shared genetic susceptibility (especially at the HLA locus). Approximately 2%–13% of coeliac disease patients have autoimmune hypothyroidism (3‐4x higher risk) and 5% have type 1 diabetes. 97 , 98 , 99 Similarly, approximately 6% (1.6%–12%) of type 1 diabetes patients develop coeliac disease. 98 Autoimmune diseases tend to cluster, for example, the risk of autoimmune thyroid disease is increased in patients with type 1 diabetes and coeliac disease compared to type 1 diabetes alone. 100 Patients with type 1 diabetes and coeliac disease have more complicated dietary requirements making dietitian input essential.
4.3. Reduced bone mineral density
There is a high prevalence of osteopenia and osteoporosis, with up to 75% of coeliac patients having reduced bone mineral density at diagnosis. 101 , 102 , 103 , 104 Symptomatic disease may be associated with more significant bone mineral loss. Traditional risk factors are not always present, so young people, those with normal BMI and males can all be affected. 104 , 105 Bone fracture risk is increased in coeliac disease 15 , 106 , 107 with the risk persisting with ongoing villous atrophy. Guidelines differ in whom and when to perform bone mineral densitometry. Testing should always be considered in patients at higher risk for bone density loss, for example long delay to diagnosis, malabsorption, severe villous atrophy at diagnosis, perimenopause or menopause in women, age >45 to 50 years in men and a history of fragility fracture. 101 , 104 Otherwise, it is reasonable to consider testing within a year of diagnosis and not later than 30–35 years of age. 5 , 104 The Fracture Risk Assessment (FRAX) tool may stratify who might benefit from bone densitometry but this approach needs further validation. 108 Five‐yearly screening is reasonable, and more frequently, if there is osteopenia or osteoporosis, evidence of ongoing disease, poor dietary adherence or refractory disease. Treatment of low bone mass with a GFD significantly improves bone density, particularly notable within the first year (approximately 5%), but fails to normalise it in all cases, especially in older patients. 109 , 110 Additional treatment includes supplementation of calcium (ideally via diet) and vitamin D and osteogenic loading (weight‐bearing) exercise. Endocrinology input should be considered if osteoporosis is present. More information is needed to inform the optimal use of anti‐resorptive drugs such as bisphosphonates in coeliac disease. Worsening bone mineral density despite a GFD should prompt assessment of the GFD and mucosal disease activity.
4.4. Liver disease
Abnormal liver function tests are a common finding in coeliac disease, with the strongest association reported at presentation or diagnosis. 111 , 112 , 113 , 114 Coeliac hepatitis is manifest by mild hypertransaminasaemia (three to five times the upper limit of normal) and is due to a gluten‐dependent liver injury that settles on a GFD. Autoimmune liver diseases such as autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis are also more common in coeliac disease. 115 , 116 , 117 An increasingly reported complication is that of non‐alcoholic fatty liver disease, 118 , 119 , 120 which can occur as part of metabolic syndrome after starting the GFD. 121 , 122 Metabolic dysfunction‐associated fatty liver disease is the revised term applied to non‐alcoholic fatty liver disease that acknowledges the spectrum of metabolic abnormalities that accompany the hepatic steatosis. The mechanism underpinning these complications is unclear. A long‐term GFD has been associated with metabolic dysregulation and cardiovascular complications, possibly through a reduced intake of whole grains 123 or higher intake of refined carbohydrates or saturated fats. 124 Coeliac patients with metabolic dysfunction‐associated fatty liver disease need strict counselling regarding increasing physical activity and optimising their diet to reduce caloric intake, enrich unprocessed, naturally gluten‐free foods and minimise highly refined carbohydrates and saturated fat. 125 Ultrasound elastography can assess for liver fibrosis.
4.5. Hyposplenism and sepsis
Patients with coeliac disease can have functional hyposplenism (12–80%) 126 , 127 , 128 that predisposes to severe sepsis from encapsulated bacteria, particularly Streptococcus pneumoniae. 129 , 130 Hyposplenism is more common with concomitant autoimmune disease, old age at coeliac diagnosis, refractory coeliac disease or a history of major infections. 127 Reduced spleen volume on imaging is a marker for refractory coeliac disease. 131 As coeliac patients who have not had pneumococcal vaccination are at increased risk of community‐acquired pneumonia, 132 vaccination against pneumococcus is recommended and vaccination against the other main encapsulated bacteria Neisseria meningitidis and Haemophilus influenzae type B should be considered. How hyposplenism screening should be undertaken and the optimal pneumococcal vaccine schedule (protein conjugate and/or polysaccharide) needs to be clarified. Patients with coeliac disease have lower responses to hepatitis B vaccination which is a particular consideration for health care workers. 133 Annual influenza vaccination and COVID‐19 vaccination are recommended.
4.6. Family screening
The risk of coeliac disease in first‐degree relatives is approximately 10%. 134 Screening symptomatic relatives for coeliac disease is strongly supported and screening asymptomatic relatives is also beneficial. 135 People who claim to be asymptomatic can have undetected issues such as low bone density and many diagnosed with coeliac disease subsequently report improved symptoms on a GFD, indicating they were never truly asymptomatic (“I didn’t realise I was sick until I felt better on a GFD”). Coeliac serology is used for screening and HLA‐DQ2/DQ8 genotyping can be added as a once‐off test to stratify the risk for coeliac disease. Positive coeliac serology should be followed up with endoscopy and biopsies to confirm the diagnosis. In family members with a negative coeliac screen, the optimal time for repeat surveillance has not been determined. In adults, every 5–10 years is reasonable, earlier if there are suggestive symptoms, and some suggest more regular screening in children to avoid the negative impact of coeliac disease on growth.
4.7. Paediatric considerations
In paediatric follow‐up, anthropometry is important to ensure appropriate growth milestones are being met. A recent large review of paediatric care in 35 countries in Europe, Israel, Turkey and Russia highlighted the need for more evidence‐based use of lab tests, increased assessment of dietary adherence and coeliac‐specific quality of life and improved attention to the transition to adult care. 12 A child that improves clinically and normalises their serology on a GFD has a low risk for persistent enteropathy. 43 Given the faster and more complete symptom resolution and mucosal healing in children compared to adults, 39 , 42 , 43 , 136 and the reluctance to perform an invasive procedure requiring deep sedation in young people, follow‐up endoscopies are not routinely performed in children. Now that a non‐biopsy‐based diagnosis of paediatric coeliac disease (applicable in select cases of high coeliac serology) is becoming established in routine practice, 137 it will be increasingly likely that many patients will not have had a diagnostic endoscopy. Persistent symptoms would warrant an endoscopy to assess treatment effects and exclude other causes.
5. NON‐RESPONSIVE/SLOWLY RESPONSIVE COELIAC DISEASE AND REFRACTORY COELIAC DISEASE
Non‐responsive coeliac disease is considered to affect up to 30% of coeliac patients on a GFD 138 , 139 and is defined as a failure to respond to at least 6 months of a GFD or the re‐emergence of symptoms, signs or laboratory abnormalities typical of coeliac disease while still following a GFD. 139 Persistent histological damage is often included in the definition, but on this criterion alone the majority of coeliac patients would be “non‐responsive” well beyond 6 months, therefore, the term typically relates to persistent symptoms. Non‐responsive coeliac disease is also common in children, with a retrospective study showing 91/616 (15%) had persistent symptoms at 6 months, with ongoing gluten ingestion (30%) or constipation (20%) the commonest causes. 140 Systematic workup identifies a cause in children and adults in most cases and patients generally improve over time. Accordingly, many favour the term “slowly responsive” disease over “non‐responsive.” 5 A suggested management approach is outlined in Figure 3.
FIGURE 3.
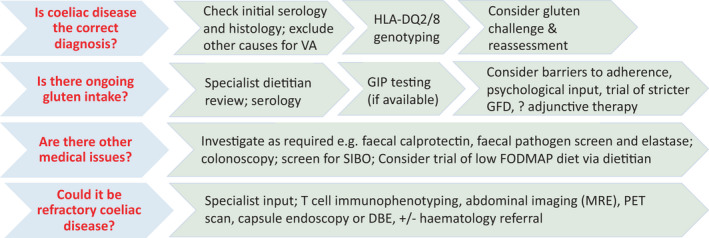
Suggested algorithm for managing non‐responsive coeliac disease. A systematic workup can generally identify a cause. Escalate investigations in the unwell, symptomatic patient until a cause is found. Refractory coeliac disease is rare but should not be overlooked. DBE, double‐balloon enteroscopy; MRE, magnetic resonance enteroscopy; PET, positron emission tomography; VA, villous atrophy
5.1. Is the diagnosis correct?
Failure to respond to a GFD may indicate an incorrect diagnosis. A review of past records is helpful to confirm the characteristic histology and positive serology at diagnosis. Negative or absent coeliac serology at diagnosis is a red flag as serology‐negative coeliac disease only constitutes a third of cases of serology‐negative villous atrophy. 141 Villous atrophy and intraepithelial lymphocytosis are not pathognomonic for coeliac disease and can have a range of causes outlined in Table 2. 141 , 142 , 143 HLA‐DQ2/DQ8 genotyping can be used to exclude coeliac disease in those with a non‐coeliac‐associated HLA genotype. In some cases, an extended gluten challenge with follow‐up assessment may be warranted to definitively confirm or exclude coeliac disease. In the future, immune‐based diagnostics may provide an alternate approach that avoids the need for gluten challenge and even endoscopy. 144
TABLE 2.
Potential causes of non‐responsive coeliac disease
| Persistent symptoms |
| Wrong diagnosis |
| Ongoing gluten ingestion |
| Irritable bowel syndrome and FODMAP intolerance, e.g lactose or fructans |
| Microscopic colitis |
| Small intestinal bacterial overgrowth |
| Pancreatic insufficiency |
| Other gastrointestinal disorders, e.g. Inflammatory bowel disease |
| Refractory coeliac disease |
| Lymphoma |
| Persistent enteropathy |
| Slowly responsive disease |
| Ongoing gluten ingestion |
| Medications associated with delayed healing: proton pump inhibitors, non‐steroidal anti‐inflammatory drugs and selective serotonin reuptake inhibitors |
| Wrong diagnosis |
| Other causes for the enteropathy |
| Immune |
| Common Variable Immunodeficiency |
| Cow’s Milk Protein Intolerance |
| Autoimmune enteropathy |
| Immune dysregulation‐related enteropathy |
| Crohn’s disease |
| Collagenous sprue |
| Graft versus host disease |
| Infective |
| Helicobacter pylori |
| Giardia lambla |
| Tropical sprue |
| Small intestinal bacterial overgrowth |
| Viral, e.g. norovirus, HIV |
| Whipple’s disease |
| Medications |
| Angiotensin receptor blocker (sartans) |
| Immune checkpoint inhibitors |
| Methotrexate |
| Mycophenolate |
| Non‐steroidal anti‐inflammatory drugs |
| Refractory coeliac disease |
| Wrong diagnosis |
5.2. Is the GFD adequate?
The commonest cause of non‐responsive coeliac disease is ongoing gluten exposure. 139 , 145 , 146 The use of GIP assays has highlighted how common inadvertent gluten ingestion is despite attempts to follow the GFD. Amounts as low as 50 mg consumed over time have been associated with intestinal damage. 147 Involving a dietitian with relevant expertise is enormously beneficial and allows a review of GFD knowledge, including how to identify sources of gluten and minimise cross‐contamination. The role of GIP assessment by doctors, dietitians and even patients to objectively assess GFD adequacy and inform changes in food choices or behaviour is promising but needs validation in clinical studies. Oats are a highly nutritious cereal that appears to be safely consumed by most people with coeliac disease if they are confirmed free of gluten contamination. 148 However, the significance of reports of oats‐induced histological damage and immune activation in coeliac disease needs to be resolved with further research. 149 , 150 , 151 Contamination‐free oats are generally allowed as part of the GFD in most countries (not Australia or New Zealand), but careful follow‐up is recommended 7 and in the setting of unexplained persistent disease activity, a trial of withholding oats may be considered.
5.3. Are there other medical causes?
There are a variety of medical issues that can cause persistent symptoms or villous damage (Table 2), and workup should be tailored to the clinical situation. Irritable bowel syndrome, food intolerances, microscopic colitis, pancreatic insufficiency, small intestinal bacterial overgrowth and refractory coeliac disease are important comorbid causes of symptoms. Persistent gastrointestinal symptoms in coeliac disease frequently resemble irritable bowel syndrome 66 , 67 and are often accompanied by malabsorption of dietary FODMAPs such as lactose, fructose or fructans (found in onion and garlic). 68 In two randomised controlled trials, dietary FODMAP reduction rapidly and significantly reduced persistent symptoms and this effect was seen even with modest FODMAP reduction. 152 , 153 Symptoms can also be caused by comorbid diseases, such as gastroparesis in type 1 diabetes and hypothyroidism or hyperthyroidisim in autoimmune thyroid disease. Reduced rates of mucosal recovery have been associated with more severe damage at diagnosis, older age and, potentially, the use of proton pump inhibitors, non‐steroidal anti‐inflammatory drugs and selective serotonin reuptake inhibitors. 74
5.4. Could it be a refractory coeliac disease?
Refractory coeliac disease is a form of complicated, non‐responsive coeliac disease that affects less than 1% of patients. 154 It is defined by persistent or recurrent malabsorptive symptoms and signs with villous atrophy despite a strict GFD for more than 12 months in the absence of other causes of non‐responsive coeliac disease and overt malignancy. 155 , 156 It is most often seen in the elderly (over 50 years) when untreated coeliac disease has been present for some time. Patients typically have a malabsorptive phenotype with diarrhoea, weight loss and lethargy. The villous atrophy is significant and can extend through to the terminal ileum and coeliac serology is often negative. GIP testing may be useful to distinguish mucosal disease due to gluten intake from true refractory coeliac disease however this approach needs validation. 157 Refractory coeliac disease is stratified into two types based on the phenotype of intestinal T cells: normal, polyclonal cells (Type 1) or aberrant, clonal cells (Type 2), based on intestinal biopsy immunohistochemistry, flow cytometry and TCR‐γ gene rearrangement studies or molecular genetics. 158 Type 1 refractory disease has a good prognosis and generally responds to immunosuppression, while type 2 refractory disease is associated with more substantial malabsorptive features (prominent symptoms, nutrient deficiencies and hypoalbuminaemia) and a poor 5‐year survival rate (<44%), with a high risk for conversion to enteropathy‐associated T cell lymphoma (50% within 5 years; 5‐year survival with enteropathy‐associated T cell lymphoma is 8%). 159 , 160 , 161
The more favourable outcome in type 1 refractory coeliac disease has prompted many experts to regard it as a relatively benign form of slowly responsive coeliac disease. Indeed, this view highlights an important limitation of how refractory coeliac disease is defined, which in its current form encompasses many patients beyond the 1% with “true” refractory disease who have some degree of persistent enteropathy and symptoms after 12 months of a strict GFD. There is a need to better distinguish true refractory disease from slowly responsive disease, perhaps via novel testing or a more stringent definition. Not surprisingly, the label “refractory coeliac disease” induces considerable patient anxiety and should be used judiciously.
When type 2 refractory coeliac disease is suspected, specialist input is crucial. MRE and PET scans are useful to assess for malignancy. Treatment aims to destroy the aberrant T‐cell clones and includes chemotherapeutic agents such as cladribine or stem cell transplantation. Identifying those at risk of developing type 2 refractory coeliac disease, better defining this complication and developing effective treatments are important unmet needs.
6. OPTIMAL MODEL OF CARE
Follow‐up of coeliac disease is inconsistent and models of care that facilitate effective, accessible and affordable care are required. In a US study, follow‐up consistent with best‐practice recommendations occurred in only 35%, while 58% had irregular follow‐up and 7% had none at all 11 ; in a UK study, 38% had no active follow‐up. 162 An Australasian study of 5310 coeliac patients showed over a third had not seen a dietitian after their diagnosis, with common information sources a patient advocacy group or the internet. 94
These deficiencies in follow‐up will be compounded in developing nations where access to doctors, dietitians, endoscopy and other medical resources are lacking, and models of follow‐up will need to be adapted and optimised to the specific region.
While data are lacking on who is best suited to provide follow‐up care, 5 , 163 gastroenterologists, dietitians and primary care doctors (general practitioners) all play important, complementary roles and models that facilitate a collaborative approach developed to work in community settings are needed. To realise such a model, there is an important need to improve the number of clinicians with expertise in coeliac disease management, including gastroenterologists and appropriately trained general practitioners. The involvement of patient advocacy groups is associated with improved GFD adherence 93 and they have an important role in reinforcing messaging to their members about the importance of medical follow‐up, which is frequently underappreciated.
A Swedish study showed long‐term care provided by the general practitioner or the gastroenterologist produces similar outcomes based on lab variables and physical and mental health scores. 164 However, patients in the general practitioner group had lower dietary adherence and were less likely to have seen a dietitian initially or at follow‐up, suggesting a need to highlight the key role of dietitians to general practitioners and facilitate meaningful associations. In Finland, most coeliac follow‐up is similarly undertaken in primary care, and attaining good adherence is achievable. 165 A UK study suggested follow‐up by a dietitian with a doctor available was most preferred by patients. 162
A general practitioner and nurse practitioner‐led telephone‐based strategy achieved many of the recommended follow‐up requirements and highlighted that proactive contacting of patients drives better engagement. 166 Regular follow‐up, even with a telephone‐based approach, improves dietary adherence. 13 , 167 These positive findings from telephone‐based follow‐up are reassuring in light of the COVID‐19 pandemic which has forced many face‐to‐face consultations to move online. As phone and telehealth consultations are likely to continue, further research to understand the benefits and limitations of these approaches for coeliac follow‐up is needed.
Given the time pressures on hospital‐based gastroenterologists and dietitians, primary care doctors could be empowered to take a central role in follow‐up, by ensuring they are provided education and consensus guidelines and access to specialist input from the dietitian and gastroenterologist. Standardising primary care follow‐up may be facilitated by innovative IT solutions, such as chronic disease management templates that can be uploaded to electronic practice software. Educating the medical profession about the importance of coeliac disease follow‐up and what best practice looks like is an ongoing need.
7. FUTURE DIRECTIONS
Current unmet needs and future directions are outlined in Figure 4. Uncertainty regarding the prognostic significance of persistent enteropathy confounds efforts to establish optimal follow‐up strategies. There is a strong case to re‐evaluate mucosal histology as the arbiter of disease activity and identify therapeutic targets associated with the reduction of long‐term complications. Coeliac disease is fundamentally an immune illness, the hallmark of which is aberrant immunity to gluten, 79 , 168 and small intestinal mucosal damage is an important but not universal feature of active disease associated with gluten‐specific immunity (an example is dermatitis herpetiformis, where enteropathy is not always present). Small intestinal histology is an imperfect “gold standard” diagnostic test, and the value of an immune (serological), non‐biopsy approach to diagnosis is now embedded in some paediatric guidelines. 137 The idea that clinically actionable information can be derived from immune data is further exemplified by serology‐positive minimal‐enteropathy coeliac disease that benefits from treatment with a GFD even when villous atrophy is absent. 169 For these reasons, an immunological measure of disease activity is an appealing candidate, however, the correlation with long‐term outcomes and the ability to translate this measure to the clinic needs to be confirmed. Currently, the only practical target that can indicate immunological remission is coeliac serology, but correlation with disease activity is suboptimal. Markers of the pathogenic gluten‐specific T cell, or related immune targets, offer promise as novel diagnostic and immunomonitoring tools and should be explored further. 144 , 170 , 171 A direct measure of gluten‐dependent immune activation would also facilitate differentiation of the enteropathy caused by coeliac disease from other causes.
FIGURE 4.
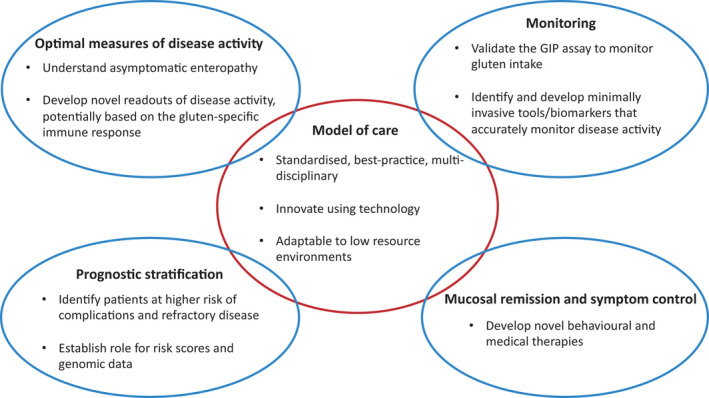
Unmet needs and future directions. Optimal models of care will underpin high‐quality and standardised patient follow‐up. This will be complemented by advances in measurement and monitoring of disease activity, the early identification of those at risk of complicated coeliac disease and novel approaches to improve mucosal healing and control symptoms
Understanding and stratifying our patients’ risk for long‐term complications or refractory coeliac disease would support a “personalised” approach to follow‐up and cost‐effective application of healthcare resources. Genetic data can inform clinical behaviour and treatment response. For example, coeliac patients who are homozygous for HLA‐DQ2.5 are more likely than their heterozygous counterparts to have severe histological and clinical disease, be slower to resolve 172 , 173 and have a higher risk of refractory coeliac disease. 174 The finer‐grained risk stratification afforded by genomic data may eventually support clinically informative prognostic scoring systems. 175 , 176
There is a major need to define the medical and behavioural interventions that can improve mucosal healing and symptom control. Therapies under development aim to reduce disease activity by quantitatively reducing gluten load and modifying gluten‐specific immunity. 50 Treatment choices may eventually be stratified by disease severity and prognostic data informing on long‐term risks. Emerging biomarkers such as interleukin‐2 show promise in differentiating gluten‐driven symptoms from other causes, 91 , 177 and an understanding of how gluten triggers symptoms and how this is linked to immune activation and mucosal disease will inform the rational design of therapies that can prevent or treat adverse symptoms.
Ultimately, improving the consistency of follow‐up management will necessitate research that informs clear, evidence‐based guidelines and effective models of care. In this way, we can ensure our patients are afforded the best opportunity to achieve optimal health outcomes.
AUTHORSHIP
Guarantor of the article: Dr Jason A. Tye‐Din.
ACKNOWLEDGEMENTS
Declaration of personal interests: JAT‐D has privately or via his institute been a consultant or advisory board member for ImmusanT Inc, Codexis, Chugai Pharmaceuticals, Genentech, IM Therapeutics, Mozart Therapeutics, Janssen, Anokion and Roche and has received research funding from Chugai Pharmaceuticals, Codexis, ImmusanT Inc., Novoviah Pharmaceuticals and Tillots Pharmaceuticals. He is an inventor on patents relating to the use of gluten peptides in coeliac disease diagnosis and treatment.
Tye‐Din J‐D. Follow‐up of coeliac disease. Aliment Pharmacol Ther. 2022;56(Suppl. 1):49–63. doi: 10.1111/apt.16847
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data in this study is based on manuscripts as listed in the bibliography.
REFERENCES
- 1. Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindfors K, Ciacci C, Kurppa K, et al. Coeliac disease. Nat Rev Dis Primers. 2019;5(1):3. [DOI] [PubMed] [Google Scholar]
- 3. See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA. Practical insights into gluten‐free diets. Nat Rev Gastroenterol Hepatol. 2015;12(10):580‐591. [DOI] [PubMed] [Google Scholar]
- 4. Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol. 2014;109(9):1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten‐related disorders. United European Gastroenterol J. 2019;7(5):583‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63(8):1210‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubio‐Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA, American College of G. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108(5):656–676; quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai JC, Ciacci C. World Gastroenterology Organisation Global guidelines: celiac disease february 2017. J Clin Gastroenterol. 2017;51(9):755‐768. [DOI] [PubMed] [Google Scholar]
- 9. Haines ML, Anderson RP, Gibson PR. Systematic review: the evidence base for long‐term management of coeliac disease. Aliment Pharmacol Ther. 2008;28(9):1042‐1066. [DOI] [PubMed] [Google Scholar]
- 10. Pinto‐Sanchez MI, Bai JC. Toward new paradigms in the follow up of adult patients with celiac disease on a gluten‐free diet. Front Nutr. 2019;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herman ML, Rubio‐Tapia A, Lahr BD, Larson JJ, Van Dyke CT, Murray JA. Patients with celiac disease are not followed up adequately. Clin Gastroenterol Hepatol. 2012;10(8):893‐899 e891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wessels M, Dolinsek J, Castillejo G, et al. Follow‐up practices for children and adolescents with celiac disease: results of an international survey. Eur J Pediatr. 2021. 10.1007/s00431-021-04318-2 [DOI] [PubMed] [Google Scholar]
- 13. Hughey JJ, Ray BK, Lee AR, Voorhees KN, Kelly CP, Schuppan D. Self‐reported dietary adherence, disease‐specific symptoms, and quality of life are associated with healthcare provider follow‐up in celiac disease. BMC Gastroenterol. 2017;17(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease‐changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156(4):885‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebwohl B, Michaelsson K, Green PH, Ludvigsson JF. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab. 2014;99(2):609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population‐based cohort study. Ann Intern Med. 2013;159(3):169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elfstrom P, Granath F, Ekstrom Smedby K, et al. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J Natl Cancer Inst. 2011;103(5):436‐444. [DOI] [PubMed] [Google Scholar]
- 18. Tio M, Cox MR, Eslick GD. Meta‐analysis: coeliac disease and the risk of all‐cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35(5):540‐551. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small‐intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302(11):1171‐1178. [DOI] [PubMed] [Google Scholar]
- 20. Lebwohl B, Green PHR, Soderling J, Roelstraete B, Ludvigsson JF. Association between celiac disease and mortality risk in a swedish population. JAMA. 2020;323(13):1277‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37(3):332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norstrom F, Lindholm L, Sandstrom O, Nordyke K, Ivarsson A. Delay to celiac disease diagnosis and its implications for health‐related quality of life. BMC Gastroenterol. 2011;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tontini GE, Rondonotti E, Saladino V, Saibeni S, de Franchis R, Vecchi M. Impact of gluten withdrawal on health‐related quality of life in celiac subjects: an observational case‐control study. Digestion. 2010;82(4):221‐228. [DOI] [PubMed] [Google Scholar]
- 24. Gray AM, Papanicolas IN. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: results from a UK population survey. BMC Health Serv Res. 2010;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Usai P, Minerba L, Marini B, et al. Case control study on health‐related quality of life in adult coeliac disease. Dig Liver Dis. 2002;34(8):547‐552. [DOI] [PubMed] [Google Scholar]
- 26. Coleman SH, Rej A, Baggus EMR, et al. What is the optimal method assessing for persistent villous atrophy in adult coeliac disease? J Gastrointestin Liver Dis. 2021;30(2):205‐212. [DOI] [PubMed] [Google Scholar]
- 27. Ludvigsson JF, Lebwohl B, Chen Q, et al. Anxiety after coeliac disease diagnosis predicts mucosal healing: a population‐based study. Aliment Pharmacol Ther. 2018;48(10):1091‐1098. [DOI] [PubMed] [Google Scholar]
- 28. Rubio‐Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten‐free diet. Am J Gastroenterol. 2010;105(6):1412‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanzini A, Lanzarotto F, Villanacci V, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten‐free diet. Aliment Pharmacol Ther. 2009;29(12):1299‐1308. [DOI] [PubMed] [Google Scholar]
- 30. Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long‐term follow‐up of celiac adults on gluten‐free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66(3):178‐185. [DOI] [PubMed] [Google Scholar]
- 31. Lee SK, Lo W, Memeo L, Rotterdam H, Green PH. Duodenal histology in patients with celiac disease after treatment with a gluten‐free diet. Gastrointest Endosc. 2003;57(2):187‐191. [DOI] [PubMed] [Google Scholar]
- 32. Bardella MT, Velio P, Cesana BM, et al. Coeliac disease: a histological follow‐up study. Histopathology. 2007;50(4):465‐471. [DOI] [PubMed] [Google Scholar]
- 33. Daveson AJM, Popp A, Taavela J, et al. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten‐free diet. GastroHep. 2020;2(1):22‐30. [Google Scholar]
- 34. Newnham ED, Shepherd SJ, Strauss BJ, Hosking P, Gibson PR. Adherence to the gluten‐free diet can achieve the therapeutic goals in almost all patients with coeliac disease: a 5‐year longitudinal study from diagnosis. J Gastroenterol Hepatol. 2016;31(2):342‐349. [DOI] [PubMed] [Google Scholar]
- 35. Sharkey LM, Corbett G, Currie E, Lee J, Sweeney N, Woodward JM. Optimising delivery of care in coeliac disease—comparison of the benefits of repeat biopsy and serological follow‐up. Aliment Pharmacol Ther. 2013;38(10):1278‐1291. [DOI] [PubMed] [Google Scholar]
- 36. Wahab PJ, Meijer JW, Mulder CJ. Histologic follow‐up of people with celiac disease on a gluten‐free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118(3):459‐463. [DOI] [PubMed] [Google Scholar]
- 37. Fernandez‐Banares F, Beltran B, Salas A, et al. Persistent villous atrophy in de novo adult patients with celiac disease and strict control of gluten‐free diet adherence: a multicenter prospective study (CADER study). Am J Gastroenterol. 2021;116(5):1036‐1043. [DOI] [PubMed] [Google Scholar]
- 38. Tuire I, Marja‐Leena L, Teea S, et al. Persistent duodenal intraepithelial lymphocytosis despite a long‐term strict gluten‐free diet in celiac disease. Am J Gastroenterol. 2012;107(10):1563‐1569. [DOI] [PubMed] [Google Scholar]
- 39. Lebwohl B, Murray JA, Rubio‐Tapia A, Green PH, Ludvigsson JF. Predictors of persistent villous atrophy in coeliac disease: a population‐based study. Aliment Pharmacol Ther. 2014;39(5):488‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hutchinson JM, West NP, Robins GG, Howdle PD. Long‐term histological follow‐up of people with coeliac disease in a UK teaching hospital. QJM. 2010;103(7):511‐517. [DOI] [PubMed] [Google Scholar]
- 41. Kreutz JM, Adriaanse MPM, van der Ploeg EMC, Vreugdenhil ACE. Narrative review: nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients. 2020;12(2): 500‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szakacs Z, Matrai P, Hegyi P, et al. Younger age at diagnosis predisposes to mucosal recovery in celiac disease on a gluten‐free diet: a meta‐analysis. PLoS One. 2017;12(11):e0187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bannister EG, Cameron DJ, Ng J, et al. Can celiac serology alone be used as a marker of duodenal mucosal recovery in children with celiac disease on a gluten‐free diet? Am J Gastroenterol. 2014;109(9):1478‐1483. [DOI] [PubMed] [Google Scholar]
- 44. Ghazzawi Y, Rubio‐Tapia A, Murray JA, Absah I. Mucosal healing in children with treated celiac disease. J Pediatr Gastroenterol Nutr. 2014;59(2):229‐231. [DOI] [PubMed] [Google Scholar]
- 45. Taavela J, Koskinen O, Huhtala H, et al. Validation of morphometric analyses of small‐intestinal biopsy readouts in celiac disease. PLoS One. 2013;8(10):e76163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ludvigsson JF, Ciacci C, Green PH, et al. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut. 2018;67(8):1410‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaukinen K, Peraaho M, Lindfors K, et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25(10):1237‐1245. [DOI] [PubMed] [Google Scholar]
- 48. Hoppe C, Gobel R, Kristensen M, et al. Intake and sources of gluten in 20‐ to 75‐year‐old Danish adults: a national dietary survey. Eur J Nutr. 2017;56(1):107‐117. [DOI] [PubMed] [Google Scholar]
- 49. Syage JA, Kelly CP, Dickason MA, et al. Determination of gluten consumption in celiac disease patients on a gluten‐free diet. Am J Clin Nutr. 2018;107(2):201‐207. [DOI] [PubMed] [Google Scholar]
- 50. Kivela L, Caminero A, Leffler DA, Pinto‐Sanchez MI, Tye‐Din JA, Lindfors K. Current and emerging therapies for coeliac disease. Nat Rev Gastroenterol Hepatol. 2021;18(3):181‐195. [DOI] [PubMed] [Google Scholar]
- 51. Silvester JA, Kurada S, Szwajcer A, Kelly CP, Leffler DA, Duerksen DR. Tests for serum transglutaminase and endomysial antibodies do not detect most patients with celiac disease and persistent villous atrophy on gluten‐free diets: a meta‐analysis. Gastroenterology. 2017;153(3):689‐701 e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ramirez‐Sanchez AD, Tan IL, Gonera‐de Jong BC, Visschedijk MC, Jonkers I, Withoff S. Molecular biomarkers for celiac disease: past, present and future. Int J Mol Sci. 2020;21(22): 8528‐8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arguelles‐Grande C, Tennyson CA, Lewis SK, Green PH, Bhagat G. Variability in small bowel histopathology reporting between different pathology practice settings: impact on the diagnosis of coeliac disease. J Clin Pathol. 2012;65(3):242‐247. [DOI] [PubMed] [Google Scholar]
- 54. Leonard MM, Cureton P, Fasano A. Indications and use of the gluten contamination elimination diet for patients with non‐responsive celiac disease. Nutrients. 2017;9(10): 1129‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mukewar SS, Sharma A, Rubio‐Tapia A, Wu TT, Jabri B, Murray JA. Open‐capsule budesonide for refractory celiac disease. Am J Gastroenterol. 2017;112(6):959‐967. [DOI] [PubMed] [Google Scholar]
- 56. Therrien A, Silvester JA, Leonard MM, Leffler DA, Fasano A, Kelly CP. Enteric‐release budesonide may be useful in the management of non‐responsive celiac disease. Dig Dis Sci. 2021;66(6):1989‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pekki H, Kurppa K, Maki M, et al. Performing routine follow‐up biopsy 1 year after diagnosis does not affect long‐term outcomes in coeliac disease. Aliment Pharmacol Ther. 2017;45(11):1459‐1468. [DOI] [PubMed] [Google Scholar]
- 58. Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten‐free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009;30(4):315‐330. [DOI] [PubMed] [Google Scholar]
- 59. Silvester JA, Comino I, Kelly CP, Sousa C, Duerksen DR, Group DBS . Most patients with celiac disease on gluten‐free diets consume measurable amounts of gluten. Gastroenterology. 2020;158(5):1497‐1499 e1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Silvester JA, Comino I, Rigaux LN, et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten‐free diet. Aliment Pharmacol Ther. 2020;52(9):1469‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stefanolo JP, Talamo M, Dodds S, et al. Real‐world gluten exposure in patients with celiac disease on gluten‐free diets, determined from gliadin immunogenic peptides in urine and fecal samples. Clin Gastroenterol Hepatol. 2021;19(3):484‐491 e481. [DOI] [PubMed] [Google Scholar]
- 62. Makharia GK, Singh P, Catassi C, et al. The global burden of coeliac disease: opportunities and challenges. Nat Rev Gastroenterol Hepatol. 2022. Epub ahead of print. 10.1038/s41575-021-00552-z [DOI] [PubMed] [Google Scholar]
- 63. Falcomer AL, Santos Araujo L, Farage P, Santos Monteiro J, Yoshio Nakano E, Puppin ZR. Gluten contamination in food services and industry: a systematic review. Crit Rev Food Sci Nutr. 2020;60(3):479‐493. [DOI] [PubMed] [Google Scholar]
- 64. Halmos EP, Clarke D, Pizzey C, Tye‐Din JA. Gluten in “gluten‐free” manufactured foods in Australia: a cross‐sectional study. Med J Aust. 2018;209(10):448‐449. [DOI] [PubMed] [Google Scholar]
- 65. Halmos EP, Di Bella CA, Webster R, Deng M, Tye‐Din JA. Gluten in “gluten‐free” food from food outlets in Melbourne: a cross‐sectional study. Med J Aust. 2018;209(1):42‐43. [DOI] [PubMed] [Google Scholar]
- 66. Silvester JA, Graff LA, Rigaux L, et al. Symptoms of functional intestinal disorders are common in patients with celiac disease following transition to a gluten‐free diet. Dig Dis Sci. 2017;62(9):2449‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome‐type symptoms in patients with celiac disease: a meta‐analysis. Clin Gastroenterol Hepatol. 2013;11(4):359‐365 e351. [DOI] [PubMed] [Google Scholar]
- 68. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67‐75 e65. [DOI] [PubMed] [Google Scholar]
- 69. Biagi F, Andrealli A, Bianchi PI, Marchese A, Klersy C, Corazza GR. A gluten‐free diet score to evaluate dietary compliance in patients with coeliac disease. Br J Nutr. 2009;102(6):882‐887. [DOI] [PubMed] [Google Scholar]
- 70. Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten‐free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7(5):530‐536. 536 e531–532, 536.e2. [DOI] [PubMed] [Google Scholar]
- 71. Lau MS, Mooney PD, White WL, et al. The role of an IgA/IgG‐deamidated gliadin peptide point‐of‐care test in predicting persistent villous atrophy in patients with celiac disease on a gluten‐free diet. Am J Gastroenterol. 2017;112(12):1859‐1867. [DOI] [PubMed] [Google Scholar]
- 72. Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten‐free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79(4):669‐673. [DOI] [PubMed] [Google Scholar]
- 73. Harder G, Schiepatti A, Biagi F, et al. Optimising the follow‐up of adult coeliac disease with a clinical‐based score to identify patients in need of a histological reassessment: a retrospective single centre study. Br J Nutr. 2020;123(10):1159‐1164. [DOI] [PubMed] [Google Scholar]
- 74. Mahadev S, Murray JA, Wu TT, et al. Factors associated with villus atrophy in symptomatic coeliac disease patients on a gluten‐free diet. Aliment Pharmacol Ther. 2017;45(8):1084‐1093. [DOI] [PubMed] [Google Scholar]
- 75. Dorn SD, Hernandez L, Minaya MT, et al. Psychosocial factors are more important than disease activity in determining gastrointestinal symptoms and health status in adults at a celiac disease referral center. Dig Dis Sci. 2010;55(11):3154‐3163. [DOI] [PubMed] [Google Scholar]
- 76. Bledsoe AC, King KS, Larson JJ, et al. Micronutrient deficiencies are common in contemporary celiac disease despite lack of overt malabsorption symptoms. Mayo Clin Proc. 2019;94(7):1253‐1260. [DOI] [PubMed] [Google Scholar]
- 77. Zanini B, Caselani F, Magni A, et al. Celiac disease with mild enteropathy is not mild disease. Clin Gastroenterol Hepatol. 2013;11(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 78. Wierdsma NJ, Bokhorst‐de V, van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5(10):3975‐3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. 2013;13(4):294‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sbravati F, Cosentino A, Lenzi J, et al. Antitissue transglutaminase antibodies' normalization after starting a gluten‐free diet in a large population of celiac children‐a real‐life experience. Dig Liver Dis. 2021. 10.1016/j.dld.2021.06.026 [DOI] [PubMed] [Google Scholar]
- 81. Bruins MJ. The clinical response to gluten challenge: a review of the literature. Nutrients. 2013;5(11):4614‐4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dipper CR, Maitra S, Thomas R, et al. Anti‐tissue transglutaminase antibodies in the follow‐up of adult coeliac disease. Aliment Pharmacol Ther. 2009;30(3):236‐244. [DOI] [PubMed] [Google Scholar]
- 83. Kaukinen K, Sulkanen S, Maki M, Collin P. IgA‐class transglutaminase antibodies in evaluating the efficacy of gluten‐free diet in coeliac disease. Eur J Gastroenterol Hepatol. 2002;14(3):311‐315. [DOI] [PubMed] [Google Scholar]
- 84. Tursi A, Brandimarte G, Giorgetti GM. Lack of usefulness of anti‐transglutaminase antibodies in assessing histologic recovery after gluten‐free diet in celiac disease. J Clin Gastroenterol. 2003;37(5):387‐391. [DOI] [PubMed] [Google Scholar]
- 85. Moreno ML, Rodriguez‐Herrera A, Sousa C, Comino I. Biomarkers to monitor gluten‐free diet compliance in celiac patients. Nutrients. 2017;9(1): 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Comino I, Fernandez‐Banares F, Esteve M, et al. Fecal gluten peptides reveal limitations of serological tests and food questionnaires for monitoring gluten‐free diet in celiac disease patients. Am J Gastroenterol. 2016;111(10):1456‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Comino I, Real A, Vivas S, et al. Monitoring of gluten‐free diet compliance in celiac patients by assessment of gliadin 33‐mer equivalent epitopes in feces. Am J Clin Nutr. 2012;95(3):670‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Costa AF, Sugai E, Temprano MP, et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J Gastroenterol. 2019;25(11):1409‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moreno ML, Cebolla A, Munoz‐Suano A, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten‐free diet and incomplete mucosal healing. Gut. 2017;66(2):250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Comino I, Segura V, Ortigosa L, et al. Prospective longitudinal study: use of faecal gluten immunogenic peptides to monitor children diagnosed with coeliac disease during transition to a gluten‐free diet. Aliment Pharmacol Ther. 2019;49(12):1484‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goel G, Tye‐Din JA, Qiao SW, et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci Adv. 2019;5(8):eaaw7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mulder CJ, Wierdsma NJ, Berkenpas M, Jacobs MA, Bouma G. Preventing complications in celiac disease: our experience with managing adult celiac disease. Best Pract Res Clin Gastroenterol. 2015;29(3):459‐468. [DOI] [PubMed] [Google Scholar]
- 93. Leffler DA, Edwards‐George J, Dennis M, et al. Factors that influence adherence to a gluten‐free diet in adults with celiac disease. Dig Dis Sci. 2008;53(6):1573‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Halmos EP, Deng M, Knowles SR, Sainsbury K, Mullan B, Tye‐Din JA. Food knowledge and psychological state predict adherence to a gluten‐free diet in a survey of 5310 Australians and New Zealanders with coeliac disease. Aliment Pharmacol Ther. 2018;48(1):78‐86. [DOI] [PubMed] [Google Scholar]
- 95. Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta‐analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update. 2014;20(4):582‐593. [DOI] [PubMed] [Google Scholar]
- 96. Canova C, Pitter G, Ludvigsson JF, Romor P, Zanier L, Zanotti R, Simonato L Celiac disease and risk of autoimmune disorders: a population‐based matched birth cohort study. J Pediatr 2016;174:146–152, e141, 146, 152.e1. [DOI] [PubMed] [Google Scholar]
- 97. Sategna‐Guidetti C, Volta U, Ciacci C, et al. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: an Italian multicenter study. Am J Gastroenterol. 2001;96(3):751‐757. [DOI] [PubMed] [Google Scholar]
- 98. Elfstrom P, Sundstrom J, Ludvigsson JF. Systematic review with meta‐analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123‐1132. [DOI] [PubMed] [Google Scholar]
- 99. Elfstrom P, Montgomery SM, Kampe O, Ekbom A, Ludvigsson JF. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab. 2008;93(10):3915‐3921. [DOI] [PubMed] [Google Scholar]
- 100. Kurien M, Mollazadegan K, Sanders DS, Ludvigsson JF. Celiac disease increases risk of thyroid disease in patients with type 1 diabetes: a nationwide cohort study. Diabetes Care. 2016;39(3):371‐375. [DOI] [PubMed] [Google Scholar]
- 101. Galli G, Lahner E, Conti L, Esposito G, Sacchi MC, Annibale B. Risk factors associated with osteoporosis in a cohort of prospectively diagnosed adult coeliac patients. United European Gastroenterol J. 2018;6(8):1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139(3):763‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pritchard L, Wilson S, Griffin J, Pearce G, Murray IA, Lewis S. Prevalence of reduced bone mineral density in adults with coeliac disease—are we missing opportunities for detection in patients below 50 years of age? Scand J Gastroenterol. 2018;53(12):1433‐1436. [DOI] [PubMed] [Google Scholar]
- 104. Fouda MA, Khan AA, Sultan MS, Rios LP, McAssey K, Armstrong D. Evaluation and management of skeletal health in celiac disease: position statement. Can J Gastroenterol. 2012;26(11):819‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mosca C, Thorsteinsdottir F, Abrahamsen B, Rumessen JJ, Handel MN. Newly diagnosed celiac disease and bone health in young adults: a systematic literature review. Calcif Tissue Int. 2022. 10.1007/s00223-021-00938-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Olmos M, Antelo M, Vazquez H, Smecuol E, Maurino E, Bai JC. Systematic review and meta‐analysis of observational studies on the prevalence of fractures in coeliac disease. Dig Liver Dis. 2008;40(1):46‐53. [DOI] [PubMed] [Google Scholar]
- 107. Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures—a general population‐based cohort study. Aliment Pharmacol Ther. 2007;25(3):273‐285. [DOI] [PubMed] [Google Scholar]
- 108. Tortora R, Imperatore N, Capone P, et al. FRAX score can be used to avoid superfluous DXA scans in detecting osteoporosis in celiac disease: accuracy of the FRAX score in celiac patients. J Clin Densitom. 2018;21(3):315‐321. [DOI] [PubMed] [Google Scholar]
- 109. Ciacci C, Maurelli L, Klain M, et al. Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. Am J Gastroenterol. 1997;92(6):992‐996. [PubMed] [Google Scholar]
- 110. Grace‐Farfaglia P. Bones of contention: bone mineral density recovery in celiac disease—a systematic review. Nutrients. 2015;7(5):3347‐3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rubio‐Tapia A, Murray JA. The liver and celiac disease. Clin Liver Dis. 2019;23(2):167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hoffmanova I, Sanchez D, Tuckova L, Tlaskalova‐Hogenova H. Celiac disease and liver disorders: from putative pathogenesis to clinical implications. Nutrients. 2018;10(7):892‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Casella G, Antonelli E, Di Bella C, et al. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int. 2013;33(7):1128‐1131. [DOI] [PubMed] [Google Scholar]
- 114. Marciano F, Savoia M, Vajro P. Celiac disease‐related hepatic injury: insights into associated conditions and underlying pathomechanisms. Dig Liver Dis. 2016;48(2):112‐119. [DOI] [PubMed] [Google Scholar]
- 115. Prasad KK, Debi U, Sinha SK, Nain CK, Singh K. Hepatobiliary disorders in celiac disease: an update. Int J Hepatol. 2011;2011:438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ludvigsson JF, Elfstrom P, Broome U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population‐based study. Clin Gastroenterol Hepatol. 2007;5(1):63‐69 e61. [DOI] [PubMed] [Google Scholar]
- 117. van Gerven NM, Bakker SF, de Boer YS, et al. Seroprevalence of celiac disease in patients with autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2014;26(10):1104‐1107. [DOI] [PubMed] [Google Scholar]
- 118. Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF. Increased risk of non‐alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015;62(6):1405‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tovoli F, Negrini G, Fari R, et al. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten‐free diet: beyond traditional metabolic factors. Aliment Pharmacol Ther. 2018;48(5):538‐546. [DOI] [PubMed] [Google Scholar]
- 120. Rispo A, Imperatore N, Guarino M, et al. Metabolic‐associated fatty liver disease (MAFLD) in coeliac disease. Liver Int. 2021;41(4):788‐798. [DOI] [PubMed] [Google Scholar]
- 121. Tortora R, Capone P, De Stefano G, et al. Metabolic syndrome in patients with coeliac disease on a gluten‐free diet. Aliment Pharmacol Ther. 2015;41(4):352‐359. [DOI] [PubMed] [Google Scholar]
- 122. Ciccone A, Gabrieli D, Cardinale R, et al. Metabolic alterations in celiac disease occurring after following a gluten‐free diet. Digestion. 2019;100(4):262‐268. [DOI] [PubMed] [Google Scholar]
- 123. Lebwohl B, Cao Y, Zong G, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ. 2017;357:j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Larussa T, Abenavoli L, Procopio AC, et al. The role of gluten‐free diet in nonalcoholic fatty liver disease development. Eur Rev Med Pharmacol Sci. 2021;25(21):6613‐6618. [DOI] [PubMed] [Google Scholar]
- 125. Aboubakr A, Stroud A, Kumar S, Newberry C. Dietary approaches for management of non‐alcoholic fatty liver disease: a clinician’s guide. Curr Gastroenterol Rep. 2021;23(12):21. [DOI] [PubMed] [Google Scholar]
- 126. Di Sabatino A, Carsetti R, Corazza GR. Post‐splenectomy and hyposplenic states. Lancet. 2011;378(9785):86‐97. [DOI] [PubMed] [Google Scholar]
- 127. Di Sabatino A, Rosado MM, Cazzola P, et al. Splenic hypofunction and the spectrum of autoimmune and malignant complications in celiac disease. Clin Gastroenterol Hepatol. 2006;4(2):179‐186. [DOI] [PubMed] [Google Scholar]
- 128. Corazza GR, Zoli G, Di Sabatino A, Ciccocioppo R, Gasbarrini G. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol. 1999;94(2):391‐397. [DOI] [PubMed] [Google Scholar]
- 129. Ludvigsson JF, Olen O, Bell M, Ekbom A, Montgomery SM. Coeliac disease and risk of sepsis. Gut. 2008;57(8):1074‐1080. [DOI] [PubMed] [Google Scholar]
- 130. Simons M, Scott‐Sheldon LAJ, Risech‐Neyman Y, Moss SF, Ludvigsson JF, Green PHR. Celiac disease and increased risk of pneumococcal infection: a systematic review and meta‐analysis. Am J Med. 2018;131(1):83‐89. [DOI] [PubMed] [Google Scholar]
- 131. van Gils T, Nijeboer P, van Waesberghe JHT, et al. Splenic volume differentiates complicated and non‐complicated celiac disease. United European Gastroenterol J. 2017;5(3):374‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zingone F, Abdul Sultan A, Crooks CJ, Tata LJ, Ciacci C, West J. The risk of community‐acquired pneumonia among 9803 patients with coeliac disease compared to the general population: a cohort study. Aliment Pharmacol Ther. 2016;44(1):57‐67. [DOI] [PubMed] [Google Scholar]
- 133. Noh KW, Poland GA, Murray JA. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol. 2003;98(10):2289‐2292. [DOI] [PubMed] [Google Scholar]
- 134. Singh P, Arora S, Lal S, Strand TA, Makharia GK. Risk of celiac disease in the first‐ and second‐degree relatives of patients with celiac disease: a systematic review and meta‐analysis. Am J Gastroenterol. 2015;110(11):1539‐1548. [DOI] [PubMed] [Google Scholar]
- 135. Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten‐free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology. 2014;147(3):610‐617 e611. [DOI] [PubMed] [Google Scholar]
- 136. Sansotta N, Amirikian K, Guandalini S, Jericho H. Celiac disease symptom resolution: effectiveness of the gluten‐free diet. J Pediatr Gastroenterol Nutr. 2018;66(1):48‐52. [DOI] [PubMed] [Google Scholar]
- 137. Husby S, Koletzko S, Korponay‐Szabo I, et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141‐156. [DOI] [PubMed] [Google Scholar]
- 138. O’Mahony S, Howdle PD, Losowsky MS. Review article: management of patients with non‐responsive coeliac disease. Aliment Pharmacol Ther. 1996;10(5):671‐680. [DOI] [PubMed] [Google Scholar]
- 139. Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5(4):445‐450. [DOI] [PubMed] [Google Scholar]
- 140. Veeraraghavan G, Therrien A, Degroote M, et al. Non‐responsive celiac disease in children on a gluten free diet. World J Gastroenterol. 2021;27(13):1311‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108(5):647‐653. [DOI] [PubMed] [Google Scholar]
- 142. Aziz I, Evans KE, Hopper AD, Smillie DM, Sanders DS. A prospective study into the aetiology of lymphocytic duodenosis. Aliment Pharmacol Ther. 2010;32(11–12):1392‐1397. [DOI] [PubMed] [Google Scholar]
- 143. Brown I, Bettington M, Rosty C. The role of histopathology in the diagnosis and management of coeliac disease and other malabsorptive conditions. Histopathology. 2021;78(1):88‐105. [DOI] [PubMed] [Google Scholar]
- 144. Smithson G, Siegelman J, Oki T, Maxwell JR, Leffler DA. The evolving landscape of biomarkers in celiac disease: leading the way to clinical development. Front Immunol. 2021;12:665756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97(8):2016‐2021. [DOI] [PubMed] [Google Scholar]
- 146. Dewar DH, Donnelly SC, McLaughlin SD, Johnson MW, Ellis HJ, Ciclitira PJ. Celiac disease: management of persistent symptoms in patients on a gluten‐free diet. World J Gastroenterol. 2012;18(12):1348‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Catassi C, Fabiani E, Iacono G, et al. A prospective, double‐blind, placebo‐controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85(1):160‐166. [DOI] [PubMed] [Google Scholar]
- 148. Pinto‐Sanchez MI, Causada‐Calo N, Bercik P, et al. Safety of adding oats to a gluten‐free diet for patients with celiac disease: systematic review and meta‐analysis of clinical and observational studies. Gastroenterology. 2017;153(2):395‐409 e393. [DOI] [PubMed] [Google Scholar]
- 149. Hardy MY, Tye‐Din JA, Stewart JA, et al. Ingestion of oats and barley in patients with celiac disease mobilizes cross‐reactive T cells activated by avenin peptides and immuno‐dominant hordein peptides. J Autoimmun. 2015;56:56‐65. [DOI] [PubMed] [Google Scholar]
- 150. Arentz‐Hansen H, Fleckenstein B, Molberg O, et al. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004;1(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lundin KE, Nilsen EM, Scott HG, et al. Oats induced villous atrophy in coeliac disease. Gut. 2003;52(11):1649‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Roncoroni L, Elli L, Doneda L, et al. A retrospective study on dietary FODMAP intake in celiac patients following a gluten‐free diet. Nutrients. 2018;10(11): 1023‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. van Megen F, Skodje GI, Lergenmuller S, et al. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms‐A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022. Epub ahead of print. 10.1016/j.cgh.2022.01.011 [DOI] [PubMed] [Google Scholar]
- 154. Ilus T, Kaukinen K, Virta LJ, et al. Refractory coeliac disease in a country with a high prevalence of clinically‐diagnosed coeliac disease. Aliment Pharmacol Ther. 2014;39(4):418‐425. [DOI] [PubMed] [Google Scholar]
- 155. Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy‐associated T‐cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356(9225):203‐208. [DOI] [PubMed] [Google Scholar]
- 156. Rubio‐Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59(4):547‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Moreno ML, Sanchez‐Munoz D, Sanders D, Rodriguez‐Herrera A, Sousa C. Verifying diagnosis of refractory celiac disease with urine gluten immunogenic peptides as biomarker. Front Med (Lausanne). 2020;7:601854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. van Gils T, Nijeboer P, van Wanrooij RL, Bouma G, Mulder CJ. Mechanisms and management of refractory coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12(10):572‐579. [DOI] [PubMed] [Google Scholar]
- 159. Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128(4 Suppl 1):S79‐S86. [DOI] [PubMed] [Google Scholar]
- 160. Mearin ML, Catassi C, Brousse N, et al. European multi‐centre study on coeliac disease and non‐Hodgkin lymphoma. Eur J Gastroenterol Hepatol. 2006;18(2):187‐194. [DOI] [PubMed] [Google Scholar]
- 161. Al‐Toma A, Verbeek WHM, Hadithi M, Von Blomberg BME, Mulder CJJ. Survival in refractory coeliac disease and enteropathy associated t‐cell lymphoma: retrospective evaluation of single‐centre experience. Gut. 2007;56(10):1373‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bebb JR, Lawson A, Knight T, Long RG. Long‐term follow‐up of coeliac disease‐‐what do coeliac patients want? Aliment Pharmacol Ther. 2006;23(6):827‐831. [DOI] [PubMed] [Google Scholar]
- 163. Kurien M, Trott N, Sanders DS. Long‐term care for patients with coeliac disease in the UK: a review of the literature and future directions. J Hum Nutr Diet. 2016;29(5):617‐623. [DOI] [PubMed] [Google Scholar]
- 164. Lexner J, Hjortswang H, Ekesbo R, Sjoberg K. Well‐being and dietary adherence in patients with coeliac disease depending on follow‐up. Scand J Gastroenterol. 2021;56(4):382‐390. [DOI] [PubMed] [Google Scholar]
- 165. Kurppa K, Lauronen O, Collin P, et al. Factors associated with dietary adherence in celiac disease: a nationwide study. Digestion. 2012;86(4):309‐314. [DOI] [PubMed] [Google Scholar]
- 166. Pritchard L, Waters C, Murray IA, Bebb J, Lewis S. Comparing alternative follow‐up strategies for patients with stable coeliac disease. Frontline Gastroenterol. 2020;11(2):93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Muhammad H, Reeves S, Ishaq S, Mayberry JF, Jeanes YM. Telephone clinic improves gluten‐free dietary adherence in adults with coeliac disease: sustained at 6 months. Frontline Gastroenterol. 2020;12(7):586‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Anderson RP. Emergence of an adaptive immune paradigm to explain celiac disease: a perspective on new evidence and implications for future interventions and diagnosis. Expert Rev Clin Immunol. 2021;18:75‐91. [DOI] [PubMed] [Google Scholar]
- 169. Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136(3):816‐823. [DOI] [PubMed] [Google Scholar]
- 170. Hardy MY, Goel G, Russell AK, et al. A sensitive whole blood assay detects antigen‐stimulated cytokine release from CD4+ T cells and facilitates immunomonitoring in a phase 2 clinical trial of Nexvax2 in coeliac disease. Front Immunol. 2021;12:661622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Leonard MM, Silvester JA, Leffler D, et al. Evaluating responses to gluten challenge: a randomized, double‐blind, 2‐dose gluten challenge trial. Gastroenterology. 2021;160(3):720‐733 e728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Karinen H, Karkkainen P, Pihlajamaki J, et al. Gene dose effect of the DQB1*0201 allele contributes to severity of coeliac disease. Scand J Gastroenterol. 2006;41(2):191‐199. [DOI] [PubMed] [Google Scholar]
- 173. Jores RD, Frau F, Cucca F, et al. HLA‐DQB1*0201 homozygosis predisposes to severe intestinal damage in celiac disease. Scand J Gastroenterol. 2007;42(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 174. Al‐Toma A, Goerres MS, Meijer JW, Pena AS, Crusius JB, Mulder CJ. Human leukocyte antigen‐DQ2 homozygosity and the development of refractory celiac disease and enteropathy‐associated T‐cell lymphoma. Clin Gastroenterol Hepatol. 2006;4(3):315‐319. [DOI] [PubMed] [Google Scholar]
- 175. Abraham G, Tye‐Din JA, Bhalala OG, Kowalczyk A, Zobel J, Inouye M. Accurate and robust genomic prediction of celiac disease using statistical learning. PLoS Genet. 2014;10(2):e1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Biagi F, Schiepatti A, Malamut G, et al. PROgnosticating COeliac patieNts SUrvivaL: the PROCONSUL score. PLoS One. 2014;9(1):e84163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Tye‐Din JA, Daveson AJM, Ee HC, et al. Elevated serum interleukin‐2 after gluten correlates with symptoms and is a potential diagnostic biomarker for coeliac disease. Aliment Pharmacol Ther. 2019;50(8):901‐910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study is based on manuscripts as listed in the bibliography.


