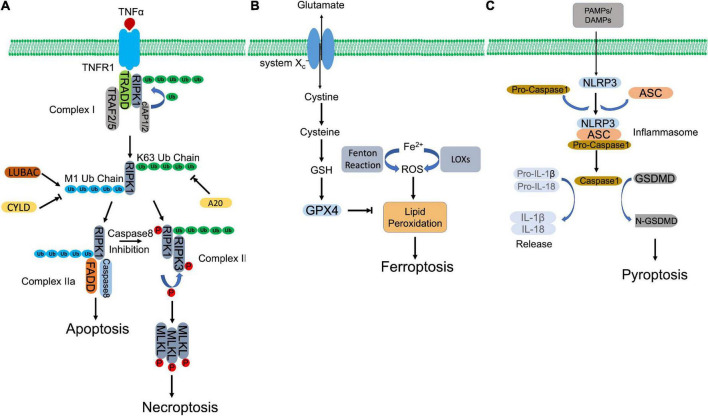
FIGURE 1.
Molecular mechanisms of regulated necrosis. (A) Necroptosis. Activation of TNFR1 by TNFα induces the formation of complex I, which contains RIPK1, TRADD, TRAF2/5, and cIAP1/2. Then, RIPK1 is ubiquitinated by cIAP1/2 to form K63-ubiquitin chains, which is removed by A20. The formed K63 uboquitination recruits LUBAC to complex I, where LUBAC catalyzes the formation of M1-ubiquitin chain on RIPK1, which is removed by CYLD. K63 ubiquitination of RIPK1 promotes the formation of necrosome, which subsequently phosphorylates MLKL to initiate necroptosis. Whereas, M1 ubiquitination induces the assembly of complex IIa, which initiates apoptosis. When caspase 8 is inhibited, necroptosis is triggered via activation of necrosome. (B) Ferroptosis. Aberrant buildup of ROS induces lipid peroxidation, which leads to ferroptosis. Intracellular ferrous ion (Fe2+) catalyzes lipid peroxidation via Fenton reaction and LOXs. GPX4, in turn, hydrolyses lipid peroxides, converting them to corresponding lipid alcohols. The antioxidant activity of GPX4 requires the participation of GSH, whose synthesis depends on cystine/glutamate antiporter system Xc–. inhibition of GPX4 or system Xc– initiates ferroptosis. (C) Pyroptosis. PAMPs and DAMPs activate inflammasome assembly. Inflammaspme sensor (NLRP3) interacts with ASC, which recruits pro-caspase 1. Pro-caspase 1 is activated by autocleavage. Activated casapse 1 not only cleaves GSDMD to induce pyroptosis, but also processes the precursors of IL-1β/IL-18 to mature IL-1β/IL-18, which are released through the pores formed by N-GSDMD.