Figure 1.
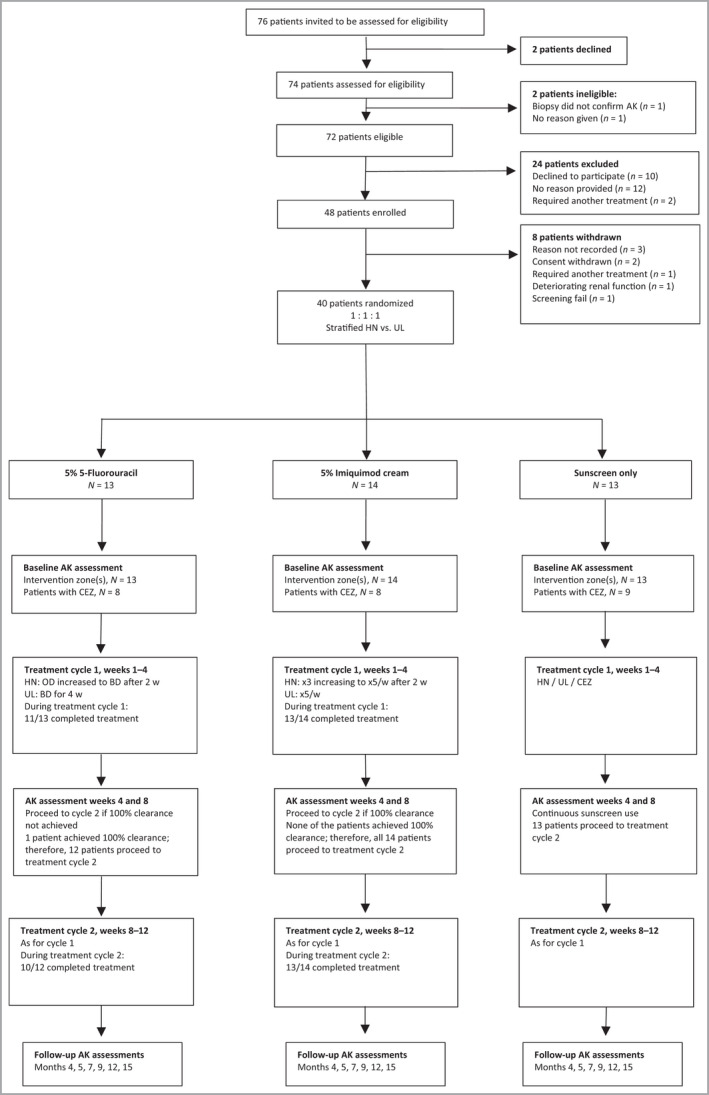
Trial design and patient flow through the study including feasibility outcomes. Of 72 eligible patients, 48 (67%, 95% CI 55–77%) agreed to participate and 40 (56%) were randomized, meeting the feasibility criterion of at least 30%. The second feasibility criterion was at least 70% of patients should complete treatment cycle 1 for the active treatment arms: this was achieved: 5‐FU 11 of 13, 85% (95% CI 55–98%); IMIQ 13 of 14, 93% (95% CI 66–100%). The third feasibility criterion was that at least 70% of patients requiring treatment should complete cycle 2 for the active treatment arms: this was achieved: [5‐FU 10 of 12, 83% (95% CI 52–98%); IMIQ 13 of 14, 93% (95% CI 66–100%)]. AK, actinic keratosis; BD, twice daily; CI, confidence interval; CEZ, clinically equivalent zone; 5‐ FU, 5% 5‐fluorouracil; HN, head and neck; IMIQ, 5% imiquimod; OD, once daily; w, week; UL, upper limbsActinic keratosis clinical assessment
