Figure 5.
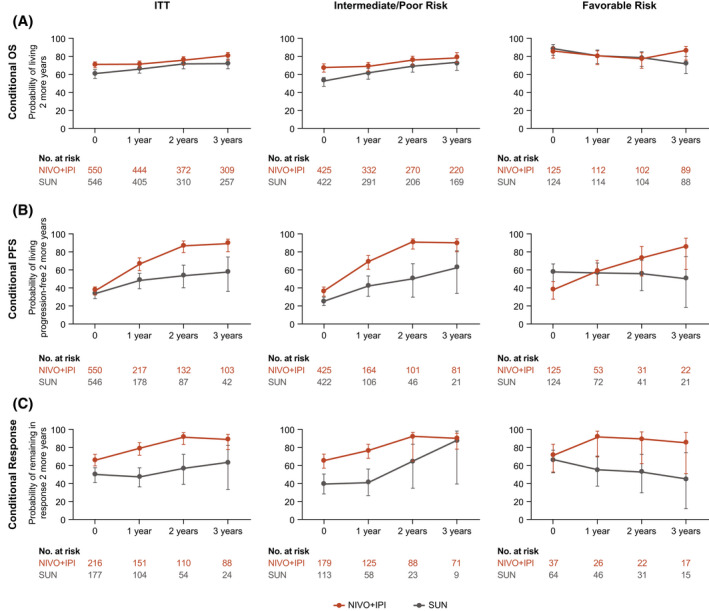
(A) Conditional overall survival (OS), (B) conditional progression‐free survival (PFS), and (C) and conditional responses are illustrated among patients in the intent‐to‐treat (ITT) group, patients who had International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate‐risk/poor‐risk disease, and patients who had IMDC favorable‐risk disease. X‐axes indicate the landmark time from randomization (conditional OS and PFS) or the landmark time from the first confirmed response (conditional response). Error bars indicate 95% confidence intervals. OS, PFS, and response probabilities were conditioned on the time alive, the time progression‐free, or the time in response after time zero. NIVO+IPI indicates nivolumab plus ipilimumab; SUN, sunitinib.
