Figure 4. In vitro therapeutic efficacy of TK-p53-NTR gene and miRs (antimiR-21, antimiR-10b, and miR-100) co-delivered using pAuNS NPs and treated using prodrugs GCV and CB1954 in TNBC cells.
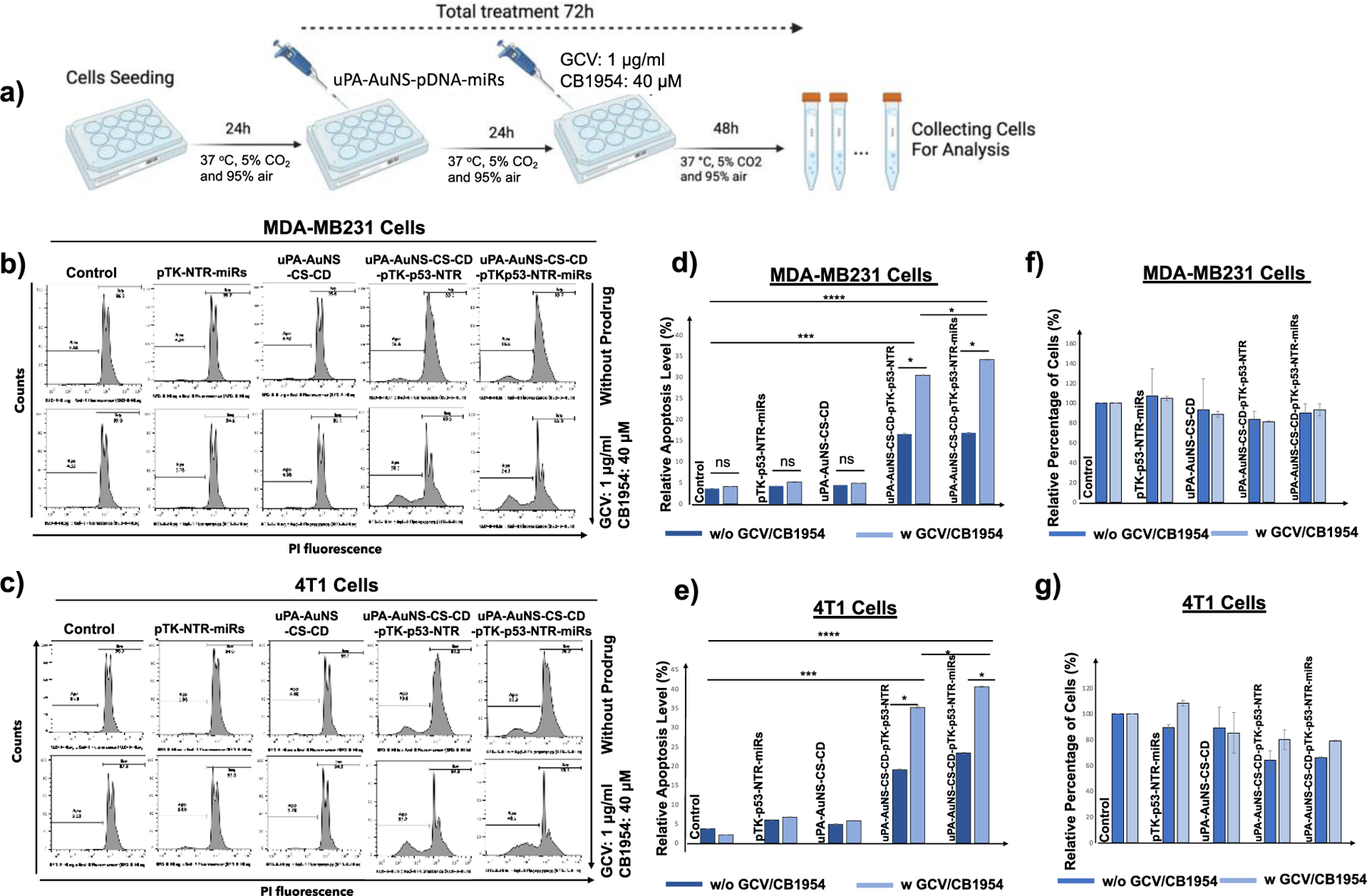
(a) Schematic illustration of TNBC cells treated with pAuNS co-loaded with miRs/TK-p53-NTR and the treatment outcome in the absence and presence of prodrugs GCV and CB1954. (b, c) Propidium Iodide (PI) staining-based FACS analyses measure the apoptotic and live cell populations in human (MDA-MB231) and mouse (4T1) TNBC cells upon treatment using empty pAuNS NPs, pAuNS loaded with TK-p53-NTR alone, miRs alone, and pAuNS co-loaded with TK-p53-NTR/miRs with and without exposure to prodrugs GCV and CB1954, and the quantitative plot of MDA-MB231 (d) and 4T1 cells (e) measured for treatment outcome. (f and g) The measured total cell numbers based on flow cytometry assay used for the quantification of cell proliferation in MDA-MB231 (f) and 4T1 (g) cells (n =3, the data were plotted as mean ± SD; Two-way ANOVA with Tukey T test and confidence interval significance was performed for comparisons; symbols indicating statistical significance were, *p< 0.05, **p< 0.01, and ***p< 0.001).
