Figure 6. Therapeutic efficacy evaluation of pAuNS NPs-mediated TK-p53-NTR/miRs co-delivery in the presence of prodrugs.
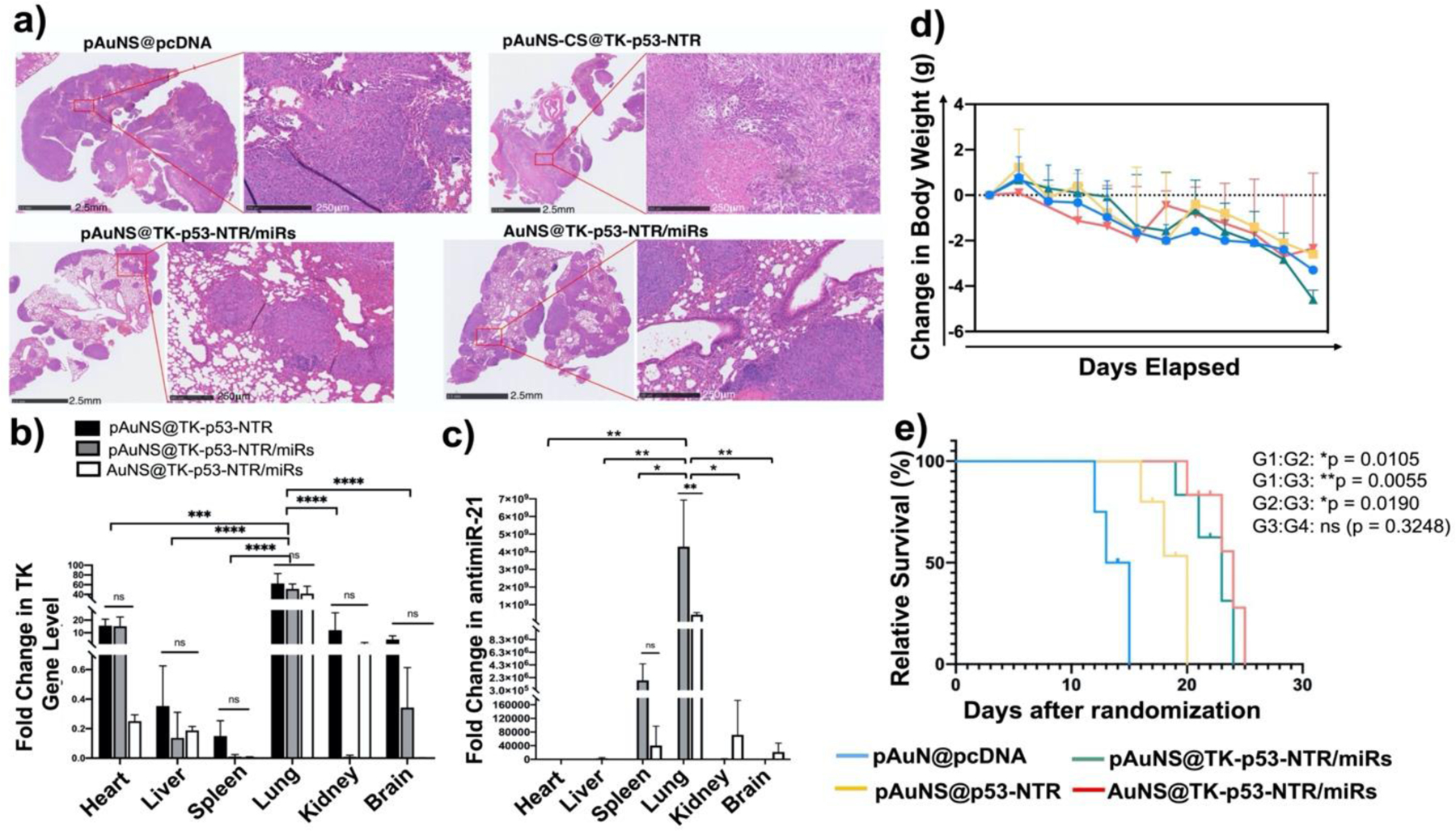
(a) Histologic analysis of lung tissue sections of different treatment groups using H&E staining assays. (b-c) Quantitative estimation of TK-p53-NTR gene and miR delivery to lungs and their biodistribution in other organs using Taqman-qPCR. (d) Bodyweight changes, and (e) survival analysis using the Gehan-Breslow-Wilcoxon method in mice during the treatment period (G1: pAuNS@pcDNA; G2: pAuNS@TK-p53-NTR; G3: pAuNS@TK-p53-NTR/miRs; G4: non-targeted AuNS@TK-p53-NTR/miRs (N=3–5/group, parts of animals were used for histological evaluations). Data are presented as mean ± SD; the significance of comparisons, as indicated, is drawn using One-way ANOVA with Bonferroni post hoc test. Adjusted p- values were considered statistically significant if p- values were < 0.05. The symbols indicating statistical significance are as follows: ns- represents non-significant difference, * represents p< 0.05, ** represents p< 0.01, *** represents p< 0.001, and **** for p< 0.0001.
