Fig. 7.
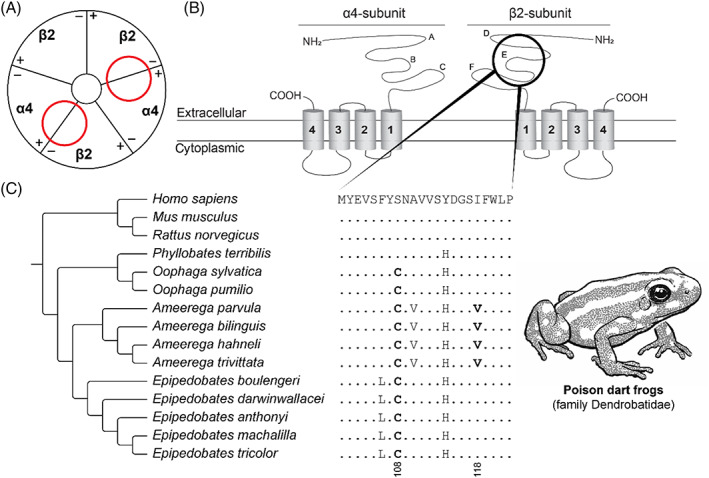
Convergent evolution of epibatidine resistance in poison dart frogs. (A) Schematic representation (based on Kini, 2019) of the neural‐type nicotinic acetylcholine receptor [nAChR; (α4)2(β2)3]. Red circles indicate the ligand‐binding domain of epibatidine in the nAChR. (B) Protein topology of the α4‐subunit and the β2‐subunit of the neural‐type nAChR. A–F indicate the loop structures at the extracellular domain in the respective subunits (Rahman et al., 2020). The black circle indicates the E‐loop involving the ligand‐binding domain of epibatidine. (C) Sequence alignment of the β2‐nAChR ligand‐binding domain. The reference amino acid sequence is from humans (Homo sapiens) and differences from this sequence are displayed for all other species. Substitutions associated with resistance are highlighted in bold. Tree topology based on Tarvin et al. (2017). For sequence accession numbers, see Table S9.
