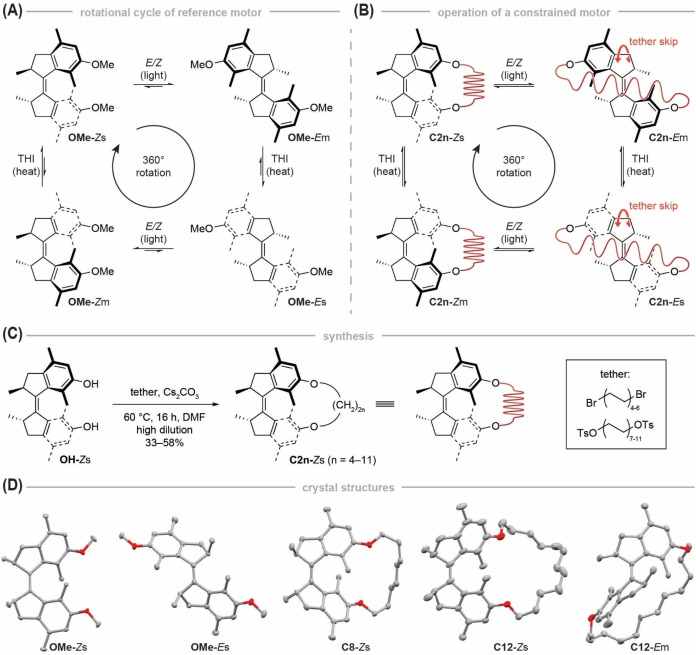
Figure 1.
Operational routine, synthesis and structural characterization of motor macrocycles. A) Full rotational cycle of unconstrained motor OMe. Starting from the Zs isomer, the motor undergoes an E/Z isomerization upon UV light illumination, followed by a subsequent THI that converts OMe ‐Em into OMe‐Es. Subsequent UV light illumination forms the Zm isomer, which undergoes another THI to form the starting isomer Zs. B) Full rotational cycle of a constrained motor C2n in which an alkyl tether functions as a molecular spring. In the Zs isomer, the tether is not strained by the motor. When illuminated with UV light, the motor is converted into the Em isomer in which the tether is subjected to chemical strain which is further increased by a subsequent THI that forms C2n ‐Es. If the tether is long enough to skip over the motor core, the system can undergo another light‐induced E/Z isomerization to form the Zm isomer, leading to a relaxation of chemical strain in the tether. Further relaxation by a THI yields the initial motor C2n‐Zs. C) Macrocyclization of dihydroxy motor OH under high‐dilution conditions by Williamson ether synthesis. D) X‐ray structures of OMe‐Zs, OMe‐Es, C8‐Zs, C12‐Zs and C12‐Em. Solvent molecules, hydrogen and disordered atoms are omitted for clarity. Ellipsoids are drawn at 50 % probability.