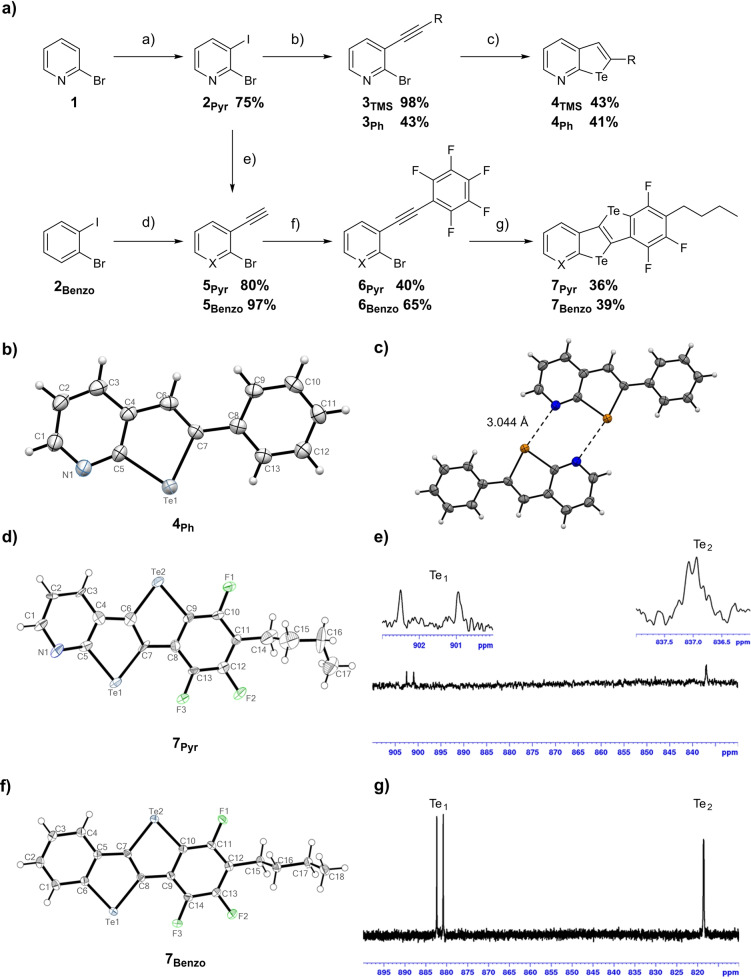
Scheme 2.
a) Synthetic pathway to 4 and 7. Reagents and conditions: a) 1. LDA, THF, −95 °C, 1 h; 2. I2, THF, −95 °C to r.t., 4 h; b) R‐≡, 5 mol% [Pd(PPh3)2Cl2], 10 mol% CuI, NEt3, 1,4‐dioxane, r.t., 1.5 h; c) 1. i‐PrBu2MgLi⋅LiCl, THF, 0 °C, 1 h; 2. Te0, r.t., 3 h; 3. EtOH, r.t., overnight; d) 1. TMSA, 5 mol% [Pd(PPh3)2Cl2], 10 mol% CuI, NEt3, 1,4‐dioxane, r.t., 1.5 h; 2. K2CO3, MeOH, CH2Cl2, r.t., 1 h; e) the same conditions as those used for (d); f) for 6Pyr : 5 mol% [Pd(PPh3)2Cl2], 10 mol% CuI, C6F5I, i‐PrNH2, toluene, 80 °C, 3 h; for 6Benzo : 5 mol% [Pd(PPh3)2Cl2], 10 mol% CuI, C6F5I, NEt3, 40 °C, overnight; g) the same conditions as those used for (c); ORTEP representation of a single molecule of b) 4Ph , d) 7Pyr and f) 7Benzo drawn with 50 % displacement ellipsoid; [32] c) crystal structure of 4Ph showing the dimer formation in the solid state; 189.5 MHz 125Te NMR spectrum in C6D6 of (e) 7Pyr and g) 7Benzo .