Figure 3.
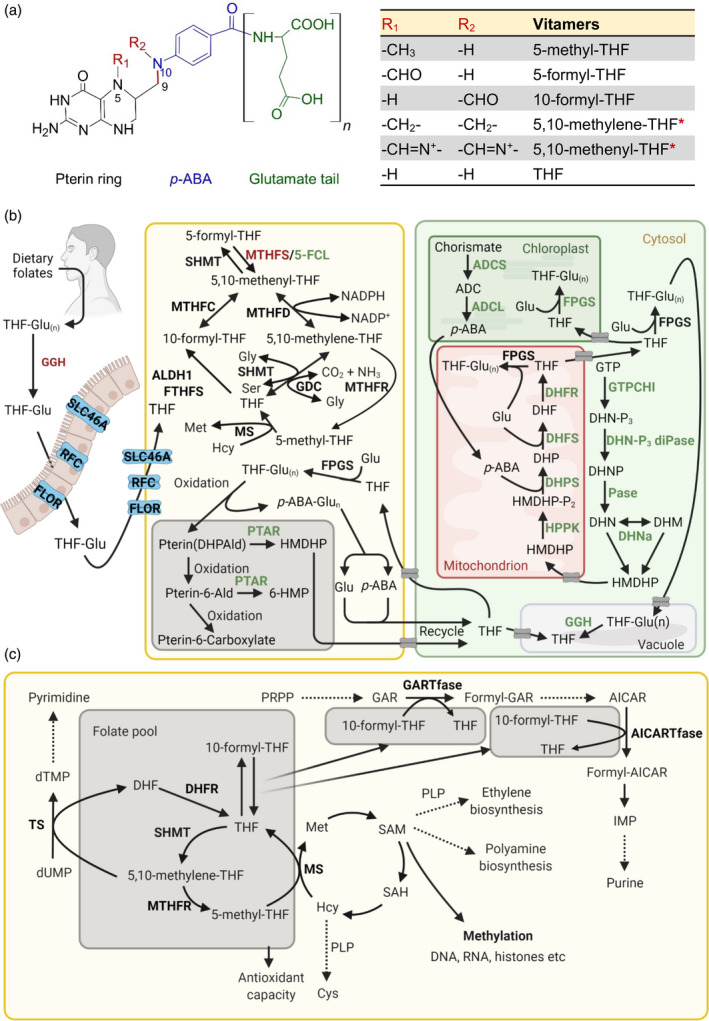
Vitamin B9 metabolism in plants and humans. (a) Generalized chemical structure of the vitamin B9 family, consisting of a pterin ring (black), p‐aminobenzoic acid (p‐ABA, blue) and one or more glutamate residues (glutamate tail, green). Vitamers differ at N5/N10, as indicated by R1 and R2, where 1C units could be attached. Vitamers listed in the table are the major coenzyme forms. The asterisks indicate linkage of the R1 and R2 groups attached at N5/N10. The C9–N10 bond (red) indicates where folate breakdown occurs, yielding pterin and p‐ABA‐glutamate (p‐ABA‐Glu(n)). (b) Biosynthesis, salvage and catabolism of vitamin B9 in plants and humans. Left: humans depend on the dietary uptake and absorption of folates, of which the tetrahydrofolate (THF) polyglutamated forms (THF‐Glu(n)) need to be converted into monoglutamate forms (THF‐Glu) by γ‐glutamyl hydrolase (GGH). Uptake, absorption and transport depend on carriers, as indicated: solute carrier family 46 member 1 (SLC46A1), reduced folate carrier (RFC) and folate receptor (FLOR), before being metabolized. Right panel: folate biosynthesis de novo in plants. pABA is biosynthesized in the plastid from chorismate via aminodeoxychorismate (ADC) by ADC synthase (ADCS) and ADC lyase (ADCL). It is then shuttled into the mitochondria for folate assembly. The pterin moiety 6‐hydroxymethyldihydropterin (HMDHP) is biosynthesized in the cytosol from guanosine triphosphate (GTP) via dihydroneopterin triphosphate (DHN‐P3), DHN phosphate (DHNP) and dihydroneopterin (DHN), catalyzed by GTP cyclohydrolase I (GTPCHI), DHN triphosphate diphosphatase (DHN‐P3 diPase) and a phosphatase (Pase). DHN can be interconverted with dihydromonapterin (DHM) by DHN aldolase (DHNa). HMDHP is then translocated into the mitochondrion and is diphosphorylated (HMDHP‐P2) by HMDHP pyrophosphokinase (HPPK). It is then conjugated with p‐ABA to form dihydropteroate (DHP), catalyzed by DHP synthase (DHPS) and then Glu to form dihydrofolate (DHF), catalyzed by DHF synthase (DHFS) and reduced to THF by DHF reductase (DHFR). Once made, folates need to be translocated into various organelles or neighbor cells to implement their respective functions. Gray boxes indicate transporters that need further investigation. Folylpolyglutamate synthase (FPGS) adds additional Glu residues in the mitochondrion, plastid or cytosol. Middle panel: salvage pathways and breakdown of folates in human and/or plants. Methylene THF reductase (MTHFR) catalyzes the conversion of 5,10‐methylene‐THF to 5‐methyl‐THF, and 5‐methyl‐THF can then be converted into THF by methionine synthase (MS), simultaneously with the conversion of homocysteine (Hcy) to methionine (Met). THF can generate 10‐formyl‐THF catalyzed by aldehyde dehydrogenase 1(ALDH1)/formyl‐THF synthetase (FTHFS). Meanwhile, THF interconverts with 5,10‐methylene‐THF, with assistance from serine hydroxymethyltransferase (SHMT) and the glycine decarboxylase complex (GDC). Additionally, conversion from 5,10‐methenyl‐THF into 10‐formyl‐THF and 5,10‐methylene‐THF requires methylene THF cyclohydrolase (MTHFC) and methylene THF dehydrogenase (MTHFD), respectively. Interconversion between 5,10‐methenyl‐THF and 5‐formyl‐THF involves SHMT, 5,10‐methenyltetrahydrofolate synthetase (MTHFS) and 5‐formyl‐THF cycloligase (5‐FCL). Folate is generally subject to oxidative cleavage in both humans and plants, yielding a pterin moiety DHP‐6‐aldehyde (DHPAld) and p‐ABA‐Glu(n). In plants, DHPAld can be converted to HMDHP by pterin aldehyde reductase (PATR) or oxidized to pterin aldehyde (pterin 6‐Ald), which can be converted to 6‐hydroxymethylpterin (6‐HMP) by PTAR or further oxidized to pterin‐6‐carboxylate. In plants (but not in humans) Glu, p‐ABA and pterin products can be recycled to assemble folate again. Also in plants THF can be stored in the vacuole in monoglutamate and polyglutamate forms, and the polyglutamate forms can be converted into THF by GGH. (c) Biochemical and physiological roles of vitamin B9. 5‐Methyl‐THF (within the folate cycle) provides the methyl group for the biosynthesis of Met from Hcy by MS (as also indicated in panel b), yielding S‐adenosyl methionine (SAM), and facilitating subsequent roles in metabolic pathways including polyamine and ethylene biosynthesis (in plants; also requires pyridoxal 5′‐phosphate (PLP)), as well as epigenetic regulation such as methylation reactions of DNA, RNA and histones. SAM can also be transformed to Hcy via S‐adenosyl‐homocysteine (SAH) to regenerate Met. Hcy is also the precursor to cysteine (Cys) via a PLP‐dependent reaction. Conversion of 10‐formyl‐THF to THF donates formyl groups for two steps in purine biosynthesis from phosphoribosylpyrophosphate (PRPP) via glycinamide ribonucleotide (GAR) transformylase (GARTfase) and 5‐aminoimidazole‐4‐carboxamide‐1‐β‐d‐ribofuranosyl 5′‐monophosphate (AICAR) transformylase (AICARTfase). In pyrimidine biosynthesis, thymidylate synthase (TS)‐catalyzed reductive methylation of deoxyuridine monophosphate (dUMP) into deoxythymidine monophosphate (dTMP) is dependent on the conversion of 5,10‐methylene‐THF to DHF. DHF can be reduced to THF by DHFR for various purposes, e.g. generating 5,10‐methylene‐THF by SHMT and subsequently 5‐methyl‐THF by MTHFR, as described in Figure 3(b). Additionally, homeostasis of the folate pool contributes to cellular redox homeostasis. Enzymes specific for plants and humans are highlighted in green and red, respectively, whereas enzymes common to both are in presented in black. The dashed lines represent sequences of multiple reactions. IMP refers to inosine monophosphate. [Colour figure can be viewed at wileyonlinelibrary.com]
