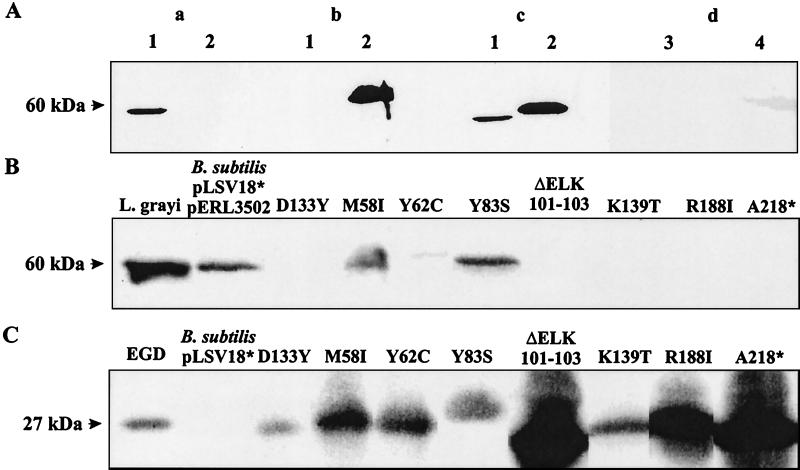
FIG. 2.
Specific recognition of p60(G) with anti-L. grayi p60 antiserum and determination of extracellular p60(G) produced by group II transformants of B. subtilis carrying pLSV18 and pERL3205. (A) Immunoblotting of culture supernatant proteins (1 ml) was performed with p60(G) antiserum (a), L. monocytogenes p60 antiserum (b), or a cocktail of both antisera (same dilutions) (c). Lanes: 1, supernatant of L. grayi; 2, supernatant of L. monocytogenes EGD. (d) Supernatants of B. subtilis DB104 (lane 3) and B. subtilis carrying pLSV18 and pERL3502 (lane 4). Proteins from supernatants (2 ml) were separated by SDS-PAGE and immunostained with the anti-p60 antisera (1:400 dilution). (B) Supernatant from the p60(G) protein of group II transformants of B. subtilis carrying pLSV18 and pERL3502. Immunoblotting of proteins from culture supernatants (2 ml) was performed with anti-p60(G) antiserum (1:400 dilution). As controls equal volumes of supernatant from L. grayi and B. subtilis pLSV18* and pERL3502 were used. pLSV18* produces an inactive p60 protein. (C) PrfA protein of the same transformants of B. subtilis carrying pLSV18 and pERL3502. Immunoblotting of bacterial lysates, separated by SDS-PAGE, was performed with PrfA antiserum (1:500 dilution). Controls are lysates from L. monocytogenes EGD (positive) and B. subtilis pLSV18* without pERL3502 (negative). The data shown are derived from differently developed immunoblots of various gel runs, which explains the different intensities of the PrfA protein bands.