Abstract
Aim
To review new challenges of fetal therapy in Japan after the establishment of four existing fetal therapies as standard prenatal care with National Health Insurance coverage over the past 20 years.
Methods
Reported studies and our current research activities related to four fetal therapies newly performed in Japan were reviewed.
Results
Fetoscopic endoluminal tracheal occlusion (FETO) for congenital diaphragmatic hernia (CDH) aims to occlude the trachea using a detachable balloon to promote lung growth. Following the recent successful completion of an international randomized controlled trial for CDH, in which we participated, FETO is offered for severe left CDH to perform balloon insertion at 27–29 weeks and removal at 34 weeks of gestation. Fetal cystoscopy (FC) for low urinary tract obstruction was introduced to overcome the demerits of vesicoamniotic shunting. FC may provide a proper diagnosis by visual observation of the urethra and physiological treatment of the posterior urethral valve. The effectiveness of open fetal surgery for myelomeningocele (MMC), direct surgery with laparotomy and hysterotomy, for ameliorating hindbrain herniation and the motor function was demonstrated, but it was also associated with substantial maternal and fetal risks. Fetal aortic valvuloplasty (FAV), ultrasound‐guided fetal aortic balloon dilation for critical aortic stenosis with evolving hypoplastic left heart syndrome may improve left heart development and maintain biventricular circulation. Feasibility and safety studies for FC, MMC open fetal surgery, and FAV are currently ongoing.
Conclusions
Clinical research on FETO, FC, MMC open fetal surgery, and FAV has proceeded with careful preparations in Japan.
Keywords: congenital diaphragmatic hernia, critical aortic stenosis, fetal therapy, low urinary tract obstruction, myelomeningocele
Introduction
Fetal therapy is medical intervention for the fetus in utero. It is performed to rescue fetuses at risk of perinatal death or severe irreversible damage, despite optimal management after birth. Fetal therapy was first attempted in the 1960s when the first fetal intrauterine blood transfusion (IUT) was performed. 1 Technologies to detect precise fetal conditions were not well advanced at that time. Advances in ultrasound imaging in the late 1970s, which enabled prenatal diagnostics, led to active attempts in fetal therapy. The concepts of “fetus as a patient” and “unborn patient” were advocated. With the accumulation of experimental and clinical experience in fetal treatment, the first conference of the International Fetal Medicine and Surgery Society (IFMSS) was held in 1982. 2 It noted that “Treatment of the fetus with a potentially correctable defect is promising but still experimental. All case material should be reported, regardless of outcome, to a fetal‐treatment registry.” This was recognized as the beginning of the modern fetal therapy.
Under ultrasound guidance, IUT for fetal anemia, 3 vesicoamniotic shunting (VAS) for fetal low urinary tract obstruction (LUTO), 4 and thoracoamniotic shunting (TAS) for fetal hydrothorax 5 were performed in the 1980s. Later, fetal aortic valvuloplasty (FAV) for critical aortic valve stenosis (CAS) 6 and radiofrequency ablation (RFA) for twin reversed arterial perfusion (TRAP) sequence 7 were also performed. Open fetal surgery, direct surgery on the fetus with hysterotomy, was performed for LUTO, 8 congenital diaphragmatic hernia (CDH), 9 and congenital pulmonary airway malformation 10 by pediatric surgeons in the US Fetoscopic laser surgery (FLS) for twin‐twin transfusion syndrome (TTTS) was started in 1990 11 and it thereafter developed into a minimally invasive percutaneous procedure that was recognized as the most successful fetal therapy. 12 , 13 , 14 A fetoscopic approach, which later developed as fetoscopic endoluminal tracheal occlusion (FETO), replaced open fetal surgery for CDH. 15 , 16 There are two trends in fetal therapy, one is moving from invasive open surgery to less invasive fetoscopic techniques, while the other is moving from clinical descriptions and retrospective analysis to randomized controlled trials. 17 Following a successful clinical trial, 18 open fetal surgery for myelomeningocele (MMC) continues.
In Japan, several attempts at fetal therapy, including IUT, 19 VAS, 20 and FLS by laparotomy for TTTS, 21 were reported in the 1980–1990s. Actual Japanese clinical research of fetal therapy began in the early 2000s with the start of FLS program for TTTS. We reported good outcomes of our initial experience of FLS for TTTS at four centers. 22 Our results confirmed that FLS was the optimal treatment option for TTTS at 16–25 weeks of gestation. They also led to the coverage of costs by Japan National Health Insurance from April 2012. FLS was the first fetal therapy to be reimbursed by Japan National Health Insurance. 23 With our favorable findings, FLS has also been applied for triplets, 24 TTTS at 26–27 weeks 25 , 26 and selective intrauterine growth restriction with oligohydramnios. 27 , 28 With the introduction of the Solomon technique and the accumulation of experience, we have achieved a very high survival rate (the at least one survival rate was >98%) 29 with favorable long‐term neurodevelopmental outcomes for TTTS treated by FLS. 30 TAS was the second fetal therapy to be compensated by Japan National Health Insurance, with the favorable results of our prospective one‐arm trial using a “double‐basket catheter” (Hakko Co., Nagano, Japan). 31 We also reported the effects of TAS on primary hydrothorax and on trisomy 21 based on a nationwide survey. 32 , 33 Owing to two studies, 34 , 35 RFA for TRAP sequence has become the third fetal therapy to be approved for coverage by Japan National Health Insurance. We have clarified the good long‐term outcomes of TRAP sequence after RFA. 36 FLS, TAS, and RFA are minimally invasive fetal therapies that have been recognized as standard prenatal care in Japan. 37 In addition, in 2020, IUT, which remains the oldest type of fetal therapy, was the fourth fetal therapy to be approved for coverage by Japan National Health Insurance based on the findings of recent studies. 38 , 39
In the past 20 years, we have been engaged in clinical research of existing fetal therapies in Japan to establish them as standard prenatal care with the approval of National Health Insurance coverage. As a result, FLS, TAS, RFA, and IUT have become standard prenatal treatments in Japan. We have proceeded to the next step to challenge new fetal therapies, including FETO for CDH, fetal cystoscopy (FC) for LUTO, open fetal surgery for MMC, and FAV for CAS. The current status of fetal surgical therapy in Japan is shown in Table 1. This review will focus on our ongoing experience of feasibility and safety studies and clinical trials for these new fetal therapies.
TABLE 1.
Current fetal surgical therapy in Japan
| 1. Fetal therapy covered by National Health Insurance (approved year) |
| Fetoscopic laser surgery for twin‐to‐twin transfusion syndrome (2012) |
| Thoracoamniotic shunting for fetal hydrothorax (2012) |
| Radiofrequency ablation for twin reversed arterial perfusion sequence (2019) |
| Intrauterine blood transfusion for fetal anemia (2020) |
| 2. Fetal therapy for which clinical trials have been completed |
| Fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia |
| 3. Fetal therapy for which feasibility and safety studies have started |
| Fetal cystoscopy for lower urinary tract obstruction |
| Fetal open surgery for meningomyelocele |
| Fetal aortic valvuloplasty for critical aortic stenosis |
FETO for CDH
CDH is a congenital disease characterized by herniation of the abdominal organs into the thoracic cavity through a diaphragm defect. 40 , 41 , 42 The prevalence of CDH is approximately 1 in 4000 live births. The overall survival rate has increased with advances in prenatal diagnostics that permit referral to experienced high volume centers, and advances in postnatal managements, especially in respiratory care. The survival rate of prenatally diagnosed isolated CDH was 79% in a nationwide survey in Japan. 43 However, the outcomes of patients with severe CDH remain poor, which denotes the limitation of postnatal treatment. Direct diaphragm repair of the fetus with laparotomy was unsuccessfully attempted, 9 then the treatment strategy moved from anatomic repair to physiological manipulations. 44 The major pathology of CDH is impairment of normal pulmonary development caused by intrathoracic herniation of the abdominal viscera. It was observed that fetal tracheal ligation promoted lung growth 45 and experimentally reversed pulmonary hypoplasia in CDH. 46 Fetal tracheal occlusion is thought to cause the accumulation alveolar fluid, which resulted in lung enlargement. Following an attempt at clipping of the trachea, 47 a surgical technique to implant a detachable balloon into the trachea under a fetoscope, named FETO, was established (Figure 1). 15 , 16
FIGURE 1.
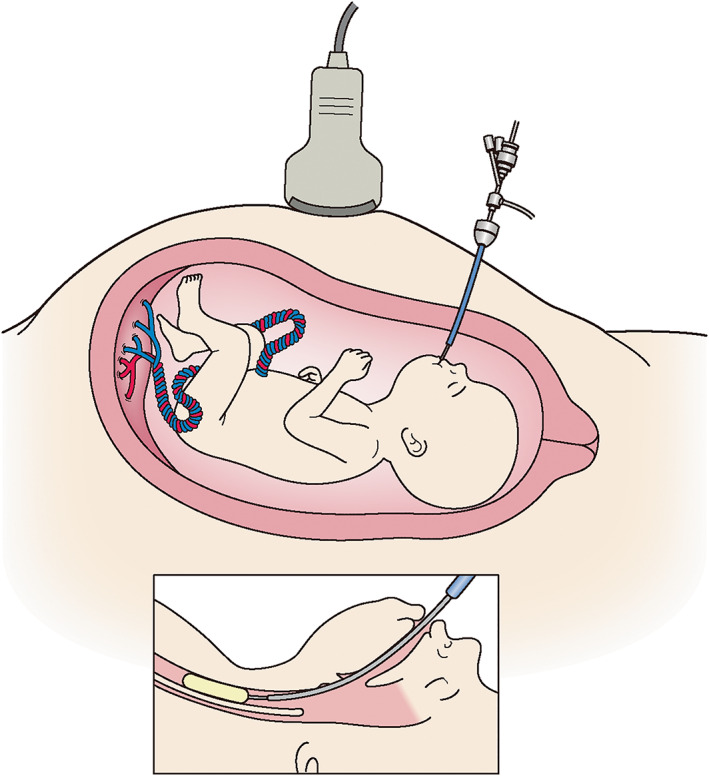
A schematic representation of fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia. A balloon is placed in the trachea through a fetoscope
Harrison et al. reported a randomized controlled trial of FETO using a 5‐mm uterine port with laparotomy for left CDH with liver herniation and a lung‐to‐head ratio (LHR) of <1.4 in 2003. 15 The results showed no improvement in survival with FETO. With many preterm birth cases in the FETO group and high survival rates in the control groups, there could be some room to improve surgical techniques and patient selection. Later, although it was a retrospective study, the European FETO Study Group reported that FETO using a percutaneous approach with a 3.3 mm cannula in left CDH with LHR <1.0 was associated with a significant survival advantage in a study population that was limited to more severe cases in comparison to previous studies. 48 , 49 These findings led to an international randomized controlled trial for CDH called the Tracheal Occlusion To Accelerate Lung Growth (TOTAL) trial.
In Japan, we initially performed clinical research on the natural history of prenatally diagnosed CDH for the selection of candidates for fetal therapy. 50 , 51 , 52 We showed that the combination of liver and stomach positions was useful to differentiate prognoses in left CDH. 52 Later, we conducted a feasibility and safety study for severe left CDH cases of Kitano G3 stomach position with intrathoracic liver herniation from 2013 to 2016. 53 Balloon insertion was performed at 27–31 weeks, and balloon removal was performed at 34 weeks. Balloon insertion was successful in all 11 cases, and balloon removal was performed at 34 weeks in all 10 cases, with the exception of 1 case that resulted in fetal death. The rate of survival to discharge was 45% (5/11), and there were no serious adverse maternal events, although there was one fetal death associated with cord strangulation. This study showed that FETO was feasible without maternal morbidity in Japan.
The TOTAL trial consisted of a protocol for severe left CDH (defined as an observed‐to‐expected LHR [o/e LHR] of <25%) to improve survival and a protocol for moderate left CDH (defined as an o/e LHR of ≥25.0% to ≤34.9%, irrespective of liver position, or an o/e LHR of ≥35.0% to ≤44.9% with intrathoracic liver herniation) to improve pulmonary complications. Balloon insertion was performed at 27–29 weeks for severe CDH and at 30–31 weeks for moderate CDH. Balloon removal was performed at 34 weeks for both severe and moderate CDH. From 2018, we participated in the TOTAL for severe CDH, which was started in 2011 and stopped early at 2020 as its efficacy was demonstrated after the third interim analysis, which included 80 women. The results for severe cases showed a significantly improved survival to discharge rate, of 40% in the FETO group in comparison to 15% in the expectant care group. 54 The rates of survival to 6 months of age were identical to the rates of survival to discharge. In contrast, the TOTAL trial for moderate CDH, which involved 196 women, started in 2008 and was completed in 2019, failed to show a significant benefit of FETO. 55 The rate of survival to discharge in moderate CDH was 63% in the FETO group and 50% in the expectant group (relative risk [RR]: 1.27. 95% confidence interval [CI]: 0.99–1.63). The rate of survival to 6 months without oxygen supplementation was 54% in the FETO group and 44% in the expectant group (RR: 1.2. 95% CI: 0.93–1.65). However, the analysis of pooled data, including these two randomized controlled trails suggests that FETO increases survival for both moderate and severe CDH. 56 Late balloon insertion for moderate CDH in the TOTAL trial is a point to be considered, although early balloon insertion increases the risk of preterm delivery. In Japan, FETO is currently offered for severe isolated left CDH (o/e LHR <25%) to perform balloon insertion at 27.0–29.9 weeks (Table 2). The balloon is removed at 34 weeks of gestation using a fetoscopic procedure, ultrasound guided puncture or ex utero intrapartum treatment. Our center is the only center in Japan that performs FETO. The establishment of one or two more additional FETO centers, reconsidering the indication criteria (including some moderate cases), and to obtaining National Health Insurance coverage are tasks to be accomplished for FETO in Japan.
TABLE 2.
Our criteria for performing FETO
|
Abbreviations: FETO, fetoscopic endoluminal tracheal occlusion; o/e LHR, observed‐to‐expected lung‐to‐head ratio.
FC for LUTO
The prevalence of LUTO is approximately 1 in 5000 births. 57 LUTO is a congenital obstructive abnormality of the urethra that could cause renal failure and bladder dysfunction. Severe cases with oligohydramnios in the first or early second trimester are associated with pulmonary hypoplasia and high neonatal mortality. 58 , 59 The characteristic ultrasound findings of LUTO are enlarged bladder (megacystis) and hydronephrosis. The etiology of LUTO includes posterior urethral valves (PUV), urethral stenosis or atresia, persistent cloaca, Prune‐belly syndrome, and megacystis microcolon intestinal hypoperistalsis syndrome. The differential diagnosis of these conditions in utero is usually difficult.
Fetuses with severe LUTO with oligohydramnios face a very poor prognosis, then fetal treatment is considered. Vesicoamniotic shunting (VAS), which continuously drains urine from the bladder to the amniotic cavity has been performed to prevent renal dysfunction and pulmonary hypoplasia in utero. In Japan, VAS has been performed using a double‐basket catheter. 20 The PLUTO study, the only randomized controlled trial of VAS, which was stopped due to poor recruitment, showed that the VAS group had a higher 2‐year survival rate in comparison to the expectant group, but there were no significant differences in the postnatal renal function. 60 A systematic review for LUTO showed that the rate of survival to 6 months of age was significantly higher in the VAS group in comparison to the expectant group, but there were no significant differences in 2‐year survival or in the postnatal renal function. 61 These results indicate that there is little evidence to support that VAS improves fetal long‐term outcomes, including the renal function.
Recently, we reported a retrospective study of 87 cases of prenatally diagnosed LUTO that were managed at two Japanese centers over 15‐year period. 62 More than half of the cases were terminated before 22 weeks of gestation and only eight cases (9%) underwent VAS. The liveborn rates in the VAS group and the conservative management group were 100% (8/8) and 56% (18/32), respectively (p = 0.034). In these groups, 6‐month survival with a normal renal function was achieved in 38% (3/8) and 16% (5/32), respectively (p = 0.608). VAS is suggested to be effective for achieving perinatal survival. PUV, a common cause of LUTO, is treatable after birth but can lead to the development of renal and pulmonary dysfunction in utero. Then, PUV is thought to be a good candidate for fetal therapy. PUV was found in one‐fifth of the cases with a confirmed diagnosis. However, most PUV cases were managed expectantly which resulted in a poor prognosis. An accurate prenatal diagnosis of LUTO, which enables the detection of PUV in utero is crucial for improving the outcomes of LUTO. Considering the limitations of ultrasound diagnosis and VAS, a new diagnostic and management strategy for LUTO is expected.
To overcome the limitations associated with ultrasound and VAS in the diagnosis and treatment of LUTO, FC was introduced in 1990s. 63 The expected advantages of FC are as follows: (1) the proper diagnosis of the etiology of LUTO by visual observation in the fetal bladder; and (2) direct treatment of the etiology which can provide more physiological urinary drainage than VAS. An accurate prenatal diagnosis could lead to appropriate counseling for patients, which will allow them to consider pregnancy outcomes. FC could treat PUV by opening the membrane using laser ablation. Ruano et al. reported a multicenter case control study of 111 fetuses with severe LUTO. The survival rates at 6 months of age in the FC, VAS, and no intervention groups were 13/34 (38%), 7/16 (44%), and 12/61 (20%), respectively. In the FC, VAS, and no intervention groups, a normal renal function was achieved in 12/16 (75%), 6/10 (60%), and 11/28 (39.3%) cases, respectively. Both FC and VAS will improve 6‐month survival; however, FC is the only treatment that may prevent renal impairment. 64 Sananes et al. reported a retrospective study of 50 LUTO cases in which FC was performed. The etiology of LUTO was diagnosed in 32/35 (91%) cases. At 2 years of age, the survival rate of patients with PUV was 54% (15/28), and 73% (11/15) showed a normal renal function. 65 FC can provide an accurate diagnosis of the cause of LUTO and the specific fetal treatment with an improved prognosis. In contrast, there was a negative report that did not show the superiority of FC in terms of long‐term outcomes, probably due to inadequate visualization of the posterior urethra. 66 FC is expected to be a promising fetal therapy; however, studies on FC have been limited.
It is necessary to examine the benefits of FC for fetuses with LUTO. We are currently conducting a safety and feasibility study of FC for the fetal diagnosis and treatment of LUTO at the Osaka Maternal and Child Medical Center and our center. The indication criteria for FC are shown in Table 3. 67 FC is performed by inserting a 1.0–1.3 mm fetal cystoscope (Karl Stortz, Tuttlingen, Germany) into the fetal bladder through the maternal abdomen under ultrasound guidance (Figure 2). Etiologies including urethral atresia or stenosis, PUV, and others are examined by observation of the internal urethral orifice from inside the bladder with investigation of the passage of water through the urethra. In the case of PUV, laser ablation using an Nd‐YAG laser is performed. This study is a single‐arm clinical trial registered as UMIN000043128 (http://www.umin.ac.jp).
TABLE 3.
Our criteria for performing FC
|
Abbreviations: FC, fetal cystoscopy; LUTO, fetal low urinary tract obstruction. and *, reference 67.
FIGURE 2.
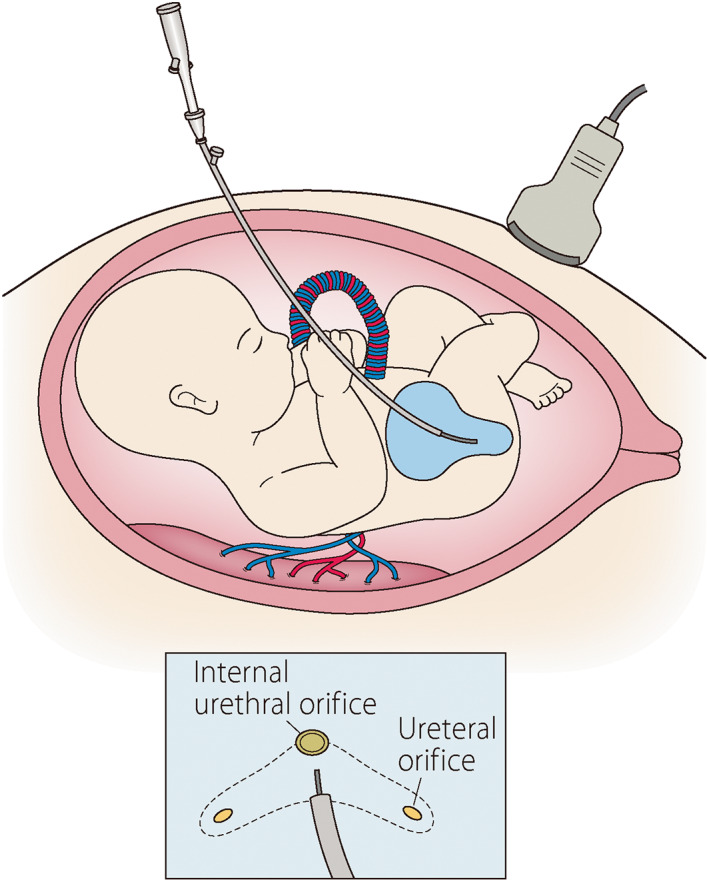
A schematic representation of fetal cystoscopy for fetal low urinary tract obstruction. To visualize the urethra and ureteral orifice and to open the valve using a laser in a case of posterior urethral valve
Fetal Surgery for MMC
MMC is a common type of spina bifida in which the spinal cord protrudes into a sac that is not covered by the skin through the spinal defect. The incidence of MMC is estimated to be 0.2–0.3 per 1000 live births in Japan. 68 At the lesion of MMC, the spinal cord is damaged, resulting in peripheral nerve injury distal to the lesion. The lesions are often located in the lumbar or sacral vertebrae resulting in limb disabilities, and bladder and rectal dysfunction. In addition, Chiari malformation type II, which is characterized by hindbrain herniation is present in almost all cases of MMC. The brainstem and cerebellum tend to herniate into the vertebral cavity due to decreased spinal cord space pressure, resulting in impairment of the circulation of cerebrospinal fluid and the central respiratory function. Children who develop hydrocephalus may have neurodevelopmental impairment. Postnatal surgery consists of repair of the meningocele early in life and ventriculo‐peritoneal shunting (V‐P shunt) for hydrocephalus afterwards. With appropriate postnatal treatment, including surgery, children with MMC are still burdened with irreversible peripheral and central neurological damage.
The pathogenesis of MMC has been proposed to consist of two stages of neurological damage, referred to the “two‐hit theory.” The “first hit” is the primary developmental abnormality due to failed closure of the neural tube. The “second hit” is the secondary neural abnormality due to chemical damage from chronic exposure to amniotic fluid and mechanical damage from contact with the uterus. The principle of fetal surgery is to reduce irreversible neural damage by preventing neural injuries caused by the “second hit.” 69 Furthermore, fetal surgery is expected to improve the hindbrain herniation, Chiari malformation type II, by suppressing the reduction of the spinal cord cavity pressure during the fetal period. Fetal surgery for MMC is the first example of the application of fetal intervention for a nonlethal condition.
Research on fetal treatment of MMC has mainly been conducted in the United States since the 1990s. In 1995, Meuli‐Simmen et al. reported that a lumbar spinal cord meningocele was surgically formed in a fetal sheep and repaired in utero to preserve the limb function. 70 In 1998, Tulipan and Bruner performed MMC repair with laparotomy and hysterotomy in four patients at 28–32 weeks of gestation and showed recovery of hindbrain herniation after birth. 71 In 1998, Adzick et al. performed fetal surgery with laparotomy and hysterotomy for thoracic to sacral MMC at 23 weeks of gestation, which resulted in the rescue of Chiari malformation type II and good developmental milestones. 72 More than 200 cases of fetal surgery for MMC were performed between 1997 and 2003. Observational studies suggested that fetal surgery could reverse hindbrain herniation with a reduced need for postnatal V‐P shunt, and improve the lower limb and bladder function. 73 , 74 , 75 They also showed increased maternal complications, including preterm PROM, preterm labor, and uterine dehiscence at the uterotomy site.
To investigate the safety and efficacy of prenatal repair of MMC, a randomized controlled trial of prenatal versus postnatal surgery for fetal MMC, named the Management of Myelomeningocele Study (MOMS), was conducted at three centers in the United States between 2003 and 2010. 18 The inclusion criteria for the study were MMC with the upper boundary located at Th1‐S1, hindbrain herniation, and gestational age 19–25 weeks. Prenatal surgery significantly reduced the composite incidence of fetal or neonatal death and V‐P shunting in comparison to postnatal surgery (68% vs. 98%, RR: 0.70, 95% CI: 0.58–0.84). In particular, the actual rate of V‐P shunting in the prenatal‐surgery group was significantly lower in comparison to the postnatal‐surgery group (40% vs. 82%, p < 0.001). Prenatal surgery was also associated with improved mental development and motor function at 30 months, hindbrain herniation by 12 months and ambulation by 30 months. However, prenatal surgery was accompanied by an increased incidence of preterm delivery, uterine incision defects, and maternal blood transfusion. MOMS shows that fetal surgery for MMC is effective for reducing V‐P shunting and improving the motor function, but is associated with substantial maternal and fetal risks.
Although open fetal surgery using laparotomy and hysterotomy has rarely been performed outside the United States, the successful results of MOMS stimulated the introduction of fetal surgery for MMC. We experienced only one case of open fetal surgery for congenital pulmonary airway malformation. 76 We started a feasibility and safety study of open fetal surgery for MMC at Osaka University Hospital and our center in 2020. The study is sponsored by the Japan Agency for Medical Research and Development and is registered as UMIN000040088 (http://www.umin.ac.jp). The indications and procedures are as same as those for MOMS (Table 4, Figure 3). We are facing difficulties in enrollment. Our nationwide survey showed that more than half of fetal MMC cases were diagnosed after 26 weeks of gestation, which is over the limit of fetal surgery and three‐quarters of cases diagnosed before 22 weeks of gestation chose termination of pregnancy. 77 In addition to knowledge of fetal surgery as a treatment option, the earlier detection of MMC by obstetric ultrasound is essential.
TABLE 4.
Our criteria for performing open fetal surgery for MMC
|
Abbreviation: MMC, myelomeningocele;
FIGURE 3.
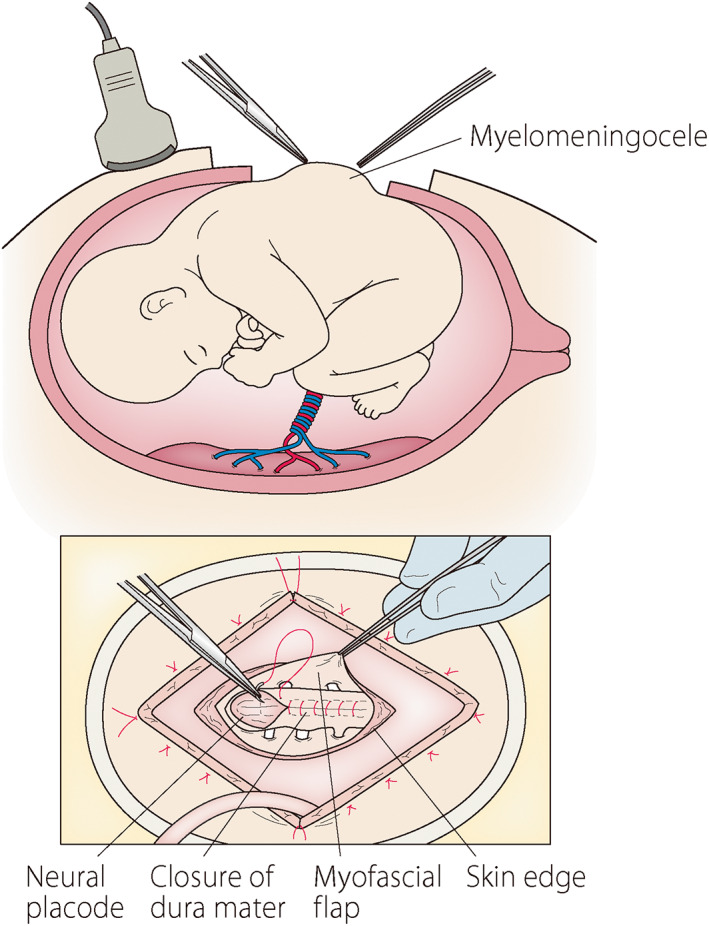
A schematic representation of open fetal surgery for myelomeningocele. The prolapsed neural placode is closed with dura mater and further covered with a myofascial flap and skin
FAV for CAS
Many congenital heart diseases can be prenatally diagnosed by fetal ultrasound. Most of structural heart diseases do not actually vary during gestation. However, some congenital heart defects could change dramatically as gestation advances, and may benefit from fetal intervention. The evolution of fetal (CAS) with enlarged left ventricle in mid‐gestation to hypoplastic left heart syndrome (HLHS) at term was reported. 78 It is thought that increased afterload due to left ventricular outflow tract stenosis leads to hypertrophy, later atrophy of the left ventricular myocardium. HLHS is characterized by mal‐development of the left‐sided structures, which hampers biventricular circulation. Advances in cardiac surgical management contribute to the improved prognosis of children with HLHS, although they still have a high risk of mortality and morbidity. 79 , 80 , 81 Fetal cardiac intervention which could improve fetal hemodynamics and may prevent the progression of mal‐development of the left heart structures.
If left ventricular outflow tract stenosis can be relieved in utero, there is a possibility of improving the development of the left ventricle and maintaining biventricular circulation after birth. Fetal cardiac intervention can be considered for CAS with evolving HLHS. Fetal cardiac intervention for CAS was first reported in 1991 by Maxwell et al. who performed ultrasound‐guided fetal aortic balloon dilation of the aortic valve in two fetuses with CAS. 6 These first two cases resulted in fetal or neonatal death. In 1995, Allan et al. reported the first case of survival. 82 In the initial 12 cases reported in the 1990s, more than half of the cases faced technical failure and only one case survived. 83 The establishment of a fetal intervention program at two centers, namely Boston Children's Hospital (USA) and The Women's and Children's Hospital Linz (Austria), in 2000 marked a turning point in fetal cardiac intervention. 84
The Women's and Children's Hospital Linz reported a total of 103 cases of CAS with evolving HLHS who underwent FAV between December 2001 and September 2020. Eighty‐seven percent of the cases were technically successful and 89% of fetuses were liveborn with a biventricular rate of 55%. 85 Boston Children's Hospital reported 143 fetuses with CAS with evolving HLHS who underwent FAV between 2000 and 2017. Eighty‐four percent of cases were technically successful; the fetal death rate was 8%. Fifty percent of the liveborn children eventually achieved biventricular circulation. 86 Taken together, approximately a technical success of 80%, fetal death rate of 10%, and biventricular circulation rate of 50% are expected following FAV for CAS with evolving HLHS.
To start the program of FAV for CAS with evolving HLHS in Japan, we conduct a feasibility and safety study on collaboration with the Japanese Society of Fetal Cardiology and Japanese Society of Pediatric Cardiology and Cardiac Surgery. The patient selection criteria consist of key echocardiographic findings to estimate the potential for evolving HLHS and a biventricular outcome (Table 5). 87 With consensus of a panel of pediatric cardiologists, FAV is performed at our Center. Under fetal anesthesia, an 18‐gauge needle is inserted into the fetal left ventricle through the thorax under ultrasound guidance. A coronary balloon catheter is introduced into the needle and placed in the aortic annulus with wire guidance and is inflated to dilate the aortic valve (Figure 4). This study is registered as UMIN000036649 (http://www.umin.ac.jp).
TABLE 5.
Our criteria for performing FAV
|
Abbreviations: FAV, fetal aortic valvuloplasty; LV, Left ventriculus.
FIGURE 4.
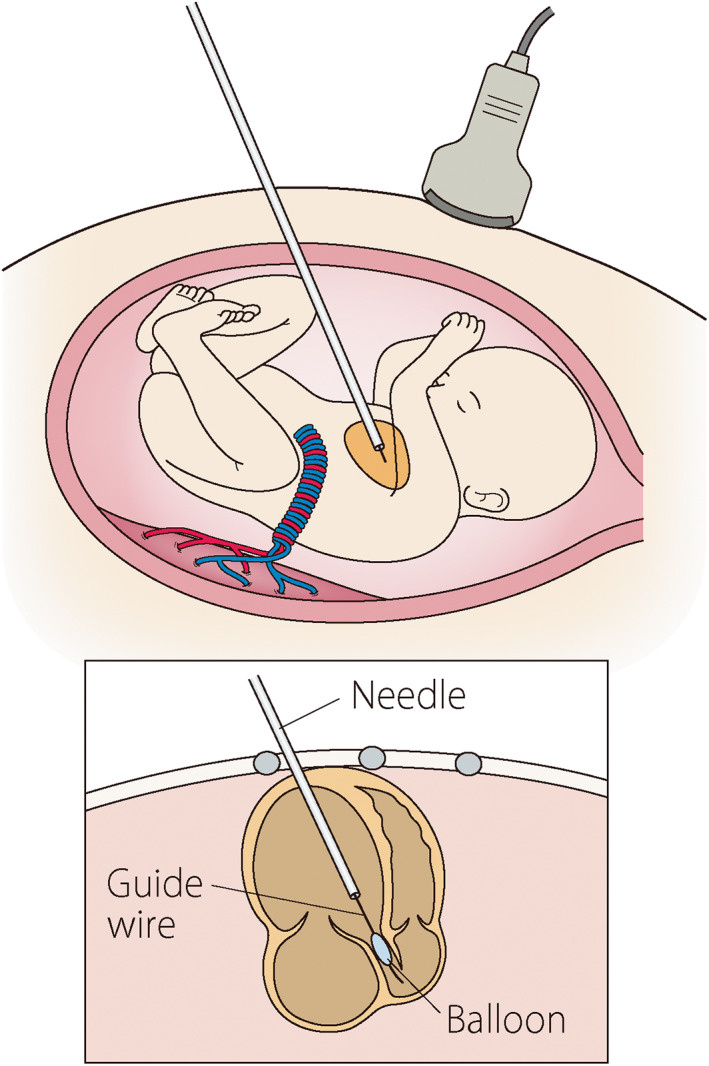
A schematic representation of fetal aortic valvuloplasty for critical aortic valve stenosis. Ultrasound‐guided dilation of the aortic valve with a guidewire and balloon
Conclusion
In Japan, new challenges for fetal therapy include FETO for CDH, FC for LUTO, open fetal surgery for MMC, and FAV for CAS, following the establishment of existing fetal therapies, and including FLS, TAS, RFA, and IUT, as standard prenatal care with National Health Insurance coverage. With the recent successful completion of an international randomized controlled trial for CDH, in which we participated, FETO is offered as a clinical treatment for severe isolated left CDH. FC for LUTO, open fetal surgery for MMC and FAV for CAS are currently being evaluated in ongoing feasibility and safety studies. We hope that these new therapies will contribute to the progress of fetal therapy, which could benefit unborn patients.
Author Contributions
All authors revised the manuscript, approved the manuscript to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
None declared.
Acknowledgments
The authors thank Dr. R. Sugibayashi and Dr. J. Muromoto for their cooperation in promoting the fetal therapy program at our center; Dr. J. A. Deprest for the establishment of the FETO program in Japan; Dr. S. Amari, Dr. Y. Ito, Dr. Y. Kanamori, Dr. H. Okuyama, Dr. N. Usui, Dr. J Sasahara, Dr. T. Kotani, Dr. M. Hayakawa, Dr. K. Kato, Dr. T. Taguchi and Dr. M. Endo for the FETO study; Dr. K. Ishii, Dr. R. Yamamoto, Dr. F. Matsui and Dr. Y. Hasegawa for FC study; Dr. M. Endo, Dr. M. Watanabe, Dr. H. Okuyama, Dr. N. Kagawa, Dr. Y. Kitabatake, Dr. H. Ogiwara and Dr. A. Flake for the MMC fetal surgery study; Dr. H. Ono and Dr. H. Kato for the FAV study. The studies of FETO, FC, and FAV were supported by the National Center for Child Health and Development of Japan under Grant numbers 2020B‐6 and 2021B‐5. The study of MMC fetal surgery was supported by AMED under Grant number JP20ek0109483.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Liley AW. Intrauterine transfusion of foetus in haemolytic disease. Br Med J. 1963;2:1107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison MR, Filly RA, Golbus MS, Berkowitz RL, Callen PW, Canty TG, et al. Fetal treatment 1982. N Engl J Med. 1982;307:1651–2. [DOI] [PubMed] [Google Scholar]
- 3. Bang J, Bock JE, Trolle D. Ultrasound‐guided fetal intravenous transfusion for severe rhesus haemolytic disease. Br Med J (Clin Res Ed). 1982;284:373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golbus MS, Harrison MR, Filly RA, Callen PW, Katz M. In utero treatment of urinary tract obstruction. Am J Obstet Gynecol. 1982;142:383–8. [DOI] [PubMed] [Google Scholar]
- 5. Rodeck CH, Fisk NM, Fraser DI, Nicolini U. Long‐term in utero drainage of fetal hydrothorax. N Engl J Med. 1988;319:1135–8. [DOI] [PubMed] [Google Scholar]
- 6. Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991;65:256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsao K, Feldstein VA, Albanese CT, Sandberg PL, Lee H, Harrison MR, et al. Selective reduction of acardiac twin by radiofrequency ablation. Am J Obstet Gynecol. 2002;187:635–40. [DOI] [PubMed] [Google Scholar]
- 8. Crombleholme TM, Harrison MR, Langer JC, Longaker MT, Anderson RL, Slotnick NS, et al. Early experience with open fetal surgery for congenital hydronephrosis. J Pediatr Surg. 1988;23:1114–21. [DOI] [PubMed] [Google Scholar]
- 9. Harrison MR, Adzick NS, Longaker MT, Goldberg JD, Rosen MA, Filly RA, et al. Successful repair in utero of a fetal diaphragmatic hernia after removal of herniated viscera from the left thorax. N Engl J Med. 1990;322:1582–4. [DOI] [PubMed] [Google Scholar]
- 10. Adzick NS, Harrison MR, Flake AW, Howell LJ, Golbus MS, Filly RA. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993;28:806–12. [DOI] [PubMed] [Google Scholar]
- 11. De Lia JE, Cruikshank DP, Keye WR Jr. Fetoscopic neodymium:YAG laser occlusion of placental vessels in severe twin‐twin transfusion syndrome. Obstet Gynecol. 1990;75:1046–53. [PubMed] [Google Scholar]
- 12. Ville Y, Hyett J, Hecher K, Nicolaides K. Preliminary experience with endoscopic laser surgery for severe twin‐twin transfusion syndrome. N Engl J Med. 1995;332:224–7. [DOI] [PubMed] [Google Scholar]
- 13. Ville Y, Hecher K, Gagnon A, Sebire N, Hyett J, Nicolaides K. Endoscopic laser coagulation in the management of severe twin‐to‐twin transfusion syndrome. Br J Obstet Gynaecol. 1998;105:446–53. [DOI] [PubMed] [Google Scholar]
- 14. Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin‐to‐twin transfusion syndrome. N Engl J Med. 2004;351:136–44. [DOI] [PubMed] [Google Scholar]
- 15. Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–24. [DOI] [PubMed] [Google Scholar]
- 16. Deprest J, Gratacos E, Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol. 2004;24:121–6. [DOI] [PubMed] [Google Scholar]
- 17. Godava M, Filipova H, Dubrava L, Vrtel R, Michalkova K, Janikova M, et al. Single giant mediastinal rhabdomyoma as a sole manifestation of TSC in foetus. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:326–9. [DOI] [PubMed] [Google Scholar]
- 18. Adzick NS, Thom EA, Spong CY, Brock JW III, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanigawara S, Okamura K, Watanabe T, et al. Intrauterine intravascular fetal transfusion under ultrasonic guide in Rh isoimmunization: a case report. Nihon Sanka Fujinka Gakkai Zasshi. 1989;41:621–4. [PubMed] [Google Scholar]
- 20. Chiba Y, Kobayashi H, Kanzaki T, Murakami M, Takahashi S, Takahashi H. Clinical aspects of fetal obstructive uropathy with in‐utero estimation of renal‐function and intrauterine shunt placement. J Matern Fetal Invest. 1993;3:225–31. [Google Scholar]
- 21. Natori M, Tanaka M, Kohno H, Ishimoto H, Morisada M, Kobayashi T, et al. A case of twin‐twin transfusion syndrome treated with placental vessel occlusion using fetoscopic Nd:YAG laser system. Nihon Sanka Fujinka Gakkai Zasshi. 1992;44:117–20. [PubMed] [Google Scholar]
- 22. Sago H, Hayashi S, Saito M, Hasegawa H, Kawamoto H, Kato N, et al. The outcome and prognostic factors of twin‐twin transfusion syndrome following fetoscopic laser surgery. Prenat Diagn. 2010;30:1185–91. [DOI] [PubMed] [Google Scholar]
- 23. Sago H, Ishii K, Sugibayashi R, Ozawa K, Sumie M, Wada S. Fetoscopic laser photocoagulation for twin‐twin transfusion syndrome. J Obstet Gynaecol Res. 2018;44:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishii K, Nakata M, Wada S, Hayashi S, Murakoshi T, Sago H. Perinatal outcome after laser surgery for triplet gestations with feto‐fetal transfusion syndrome. Prenat Diagn. 2014;34:734–8. [DOI] [PubMed] [Google Scholar]
- 25. Nakata M, Ishii K, Sumie M, Takano M, Hirata H, Murata S, et al. A prospective pilot study of fetoscopic laser surgery for twin‐to‐twin transfusion syndrome between 26 and 27 weeks of gestation. Taiwan J Obstet Gynecol. 2016;55:512–4. [DOI] [PubMed] [Google Scholar]
- 26. Takano M, Nakata M, Ishii K, Wada S, Sumie M, Yamamoto R, et al. Outcomes of fetoscopic laser surgery for twin‐to‐twin transfusion syndrome between 26 and 27 weeks of gestation in Japan. J Obstet Gynaecol Res. 2021;47:3821–7. [DOI] [PubMed] [Google Scholar]
- 27. Ishii K, Nakata M, Wada S, Murakoshi T, Sago H. Feasibility and preliminary outcomes of fetoscopic laser photocoagulation for monochorionic twin gestation with selective intrauterine growth restriction accompanied by severe oligohydramnios. J Obstet Gynaecol Res. 2015;41:1732–7. [DOI] [PubMed] [Google Scholar]
- 28. Ishii K, Wada S, Takano M, Nakata M, Murakoshi T, Sago H. Survival rate without brain abnormalities on postnatal ultrasonography among Monochorionic twins after Fetoscopic laser photocoagulation for selective intrauterine growth restriction with concomitant oligohydramnios. Fetal Diagn Ther. 2019;45:21–7. [DOI] [PubMed] [Google Scholar]
- 29. Kanazawa S, Ozawa K, Muromoto J, Sugibayashi R, Wada Y, Wada S, et al. Risk profiling of the Solomon technique versus selective technique of Fetoscopic laser surgery for twin‐twin transfusion syndrome. Twin Res Hum Genet. 2021;24:42–8. [DOI] [PubMed] [Google Scholar]
- 30. Matsushima S, Ozawa K, Sugibayashi R, Ogawa K, Tsukamoto K, Miyazaki O, et al. Neurodevelopmental impairment at 3 years of age after fetoscopic laser surgery for twin‐to‐twin transfusion syndrome. Prenat Diagn. 2020;40:1013–9. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi Y, Kawabata I, Sumie M, Nakata M, Ishii K, Murakoshi T, et al. Thoracoamniotic shunting for fetal pleural effusions using a double‐basket shunt. Prenat Diagn. 2012;32:1282–7. [DOI] [PubMed] [Google Scholar]
- 32. Wada S, Jwa SC, Yumoto Y, Takahashi Y, Ishii K, Usui N, et al. The prognostic factors and outcomes of primary fetal hydrothorax with the effects of fetal intervention. Prenat Diagn. 2017;37:184–92. [DOI] [PubMed] [Google Scholar]
- 33. Yumoto Y, Jwa SC, Wada S, Takahashi Y, Ishii K, Kato K, et al. The outcomes and prognostic factors of fetal hydrothorax associated with trisomy 21. Prenat Diagn. 2017;37:686–92. [DOI] [PubMed] [Google Scholar]
- 34. Sugibayashi R, Ozawa K, Sumie M, Wada S, Ito Y, Sago H. Forty cases of twin reversed arterial perfusion sequence treated with radio frequency ablation using the multistep coagulation method: a single‐center experience. Prenat Diagn. 2016;36:437–43. [DOI] [PubMed] [Google Scholar]
- 35. Wagata M, Murakoshi T, Ishii K, Muromoto J, Sasahara J, Murotsuki J. Radiofrequency ablation with an internally cooled electrode for twin reversed arterial perfusion sequence. Fetal Diagn Ther. 2016;40:110–5. [DOI] [PubMed] [Google Scholar]
- 36. Ozawa K, Wada S, Muromoto J, Sugibayashi R, Wada YS, Ito Y, et al. Long‐term neurodevelopmental outcomes of the pump twin in twin reversed arterial perfusion sequence treated by radiofrequency ablation. Prenat Diagn. 2021;41:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sago H, Wada S. Fetal therapies as standard prenatal care in Japan. Obstet Gynecol Sci. 2020;63:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sasahara J, Ishii K, Fujikawa E, Mitsuda N. Current status of percutaneous umbilical cord blood sampling in Japan. J Obstet Gynaecol Res. 2019;45:1821–7. [DOI] [PubMed] [Google Scholar]
- 39. Mizuuchi M, Murotsuki J, Ishii K, Yamamoto R, Sasahara J, Wada S, et al. Nationwide survey of intrauterine blood transfusion for fetal anemia in Japan. J Obstet Gynaecol Res. 2021;47:2076–81. [DOI] [PubMed] [Google Scholar]
- 40. Hedrick HL. Evaluation and management of congenital diaphragmatic hernia. Pediatr Case Rev. 2001;1:25–36. [DOI] [PubMed] [Google Scholar]
- 41. Gallot D, Boda C, Ughetto S, Perthus I, Robert‐Gnansia E, Francannet C, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry‐based study. Ultrasound Obstet Gynecol. 2007;29:276–83. [DOI] [PubMed] [Google Scholar]
- 42. Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–5. [DOI] [PubMed] [Google Scholar]
- 43. Nagata K, Usui N, Kanamori Y, Takahashi S, Hayakawa M, Okuyama H, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48:738–44. [DOI] [PubMed] [Google Scholar]
- 44. Jancelewicz T, Harrison MR. A history of fetal surgery. Clin Perinatol. 2009;36(227–36):vii–236. [DOI] [PubMed] [Google Scholar]
- 45. Carmel JA, Friedman F, Adams FH. Fetal tracheal ligation and lung development. Am J Dis Child. 1965;109:452–6. [DOI] [PubMed] [Google Scholar]
- 46. DiFiore JW, Fauza DO, Slavin R, Peters CA, Fackler JC, Wilson JM. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:248–56; discussion 256‐7. [DOI] [PubMed] [Google Scholar]
- 47. Harrison MR, Adzick NS, Bullard KM, Farrell JA, Howell LJ, Rosen MA, et al. Correction of congenital diaphragmatic hernia in utero VII: a prospective trial. J Pediatr Surg. 1997;32:1637–42. [DOI] [PubMed] [Google Scholar]
- 48. Doné E, Gratacos E, Nicolaides KH, Allegaert K, Valencia C, Castañon M, et al. Predictors of neonatal morbidity in fetuses with severe isolated congenital diaphragmatic hernia undergoing fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2013;42:77–83. [DOI] [PubMed] [Google Scholar]
- 49. Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:304–10. [DOI] [PubMed] [Google Scholar]
- 50. Okuyama H, Kitano Y, Saito M, Usui N, Morikawa N, Masumoto K, et al. The Japanese experience with prenatally diagnosed congenital diaphragmatic hernia based on a multi‐institutional review. Pediatr Surg Int. 2011;27:373–8. [DOI] [PubMed] [Google Scholar]
- 51. Usui N, Kitano Y, Okuyama H, Saito M, Morikawa N, Takayasu H, et al. Reliability of the lung to thorax transverse area ratio as a predictive parameter in fetuses with congenital diaphragmatic hernia. Pediatr Surg Int. 2011;27:39–45. [DOI] [PubMed] [Google Scholar]
- 52. Kitano Y, Okuyama H, Saito M, Usui N, Morikawa N, Masumoto K, et al. Re‐evaluation of stomach position as a simple prognostic factor in fetal left congenital diaphragmatic hernia: a multicenter survey in Japan. Ultrasound Obstet Gynecol. 2011;37:277–82. [DOI] [PubMed] [Google Scholar]
- 53. Wada S, Ozawa K, Sugibayashi R, Suyama F, Endo M, Sago H. Feasibility and outcomes of fetoscopic endoluminal tracheal occlusion for severe congenital diaphragmatic hernia: a Japanese experience. J Obstet Gynaecol Res. 2020;46:2598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. 2021;385:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med. 2021;385:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Calster B, Benachi A, Nicolaides KH, et al. The randomized TOTAL‐trials on fetal surgery for congenital diaphragmatic hernia: re‐analysis using pooled data. Am J Obstet Gynecol. 2022;226(4):560.e1–560.e24. [DOI] [PubMed] [Google Scholar]
- 57. Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenat Diagn. 2005;25:7–13. [DOI] [PubMed] [Google Scholar]
- 58. Ruano R. Fetal surgery for severe lower urinary tract obstruction. Prenat Diagn. 2011;31:667–74. [DOI] [PubMed] [Google Scholar]
- 59. Taghavi K, Sharpe C, Stringer MD. Fetal megacystis: a systematic review. J Pediatr Urol. 2017;13:7–15. [DOI] [PubMed] [Google Scholar]
- 60. Morris RK, Malin GL, Quinlan‐Jones E, Middleton LJ, Hemming K, Burke D, et al. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet. 2013;382:1496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saccone G, D'Alessandro P, Escolino M, et al. Antenatal intervention for congenital fetal lower urinary tract obstruction (LUTO): a systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2020;33:2664–70. [DOI] [PubMed] [Google Scholar]
- 62. Sugibayashi R, Wada S, Ozawa K, Muromoto J, Oi R, Yamamoto R, et al. Prenatally diagnosed lower urinary tract obstruction: a 15‐year experience at two tertiary centers in Japan. J Obstet Gynaecol Res. 2021;47:3091–9. [DOI] [PubMed] [Google Scholar]
- 63. Quintero RA, Johnson MP, Romero R, Cotton DB, Evans MI, Smith C, et al. In‐utero percutaneous cystoscopy in the management of fetal lower obstructive uropathy. Lancet. 1995;346:537–40. [DOI] [PubMed] [Google Scholar]
- 64. Ruano R, Sananes N, Sangi‐Haghpeykar H, Hernandez‐Ruano S, Moog R, Becmeur F, et al. Fetal intervention for severe lower urinary tract obstruction: a multicenter case‐control study comparing fetal cystoscopy with vesicoamniotic shunting. Ultrasound Obstet Gynecol. 2015;45:452–8. [DOI] [PubMed] [Google Scholar]
- 65. Sananes N, Cruz‐Martinez R, Favre R, Ordorica‐Flores R, Moog R, Zaloszy A, et al. Two‐year outcomes after diagnostic and therapeutic fetal cystoscopy for lower urinary tract obstruction. Prenat Diagn. 2016;36:297–303. [DOI] [PubMed] [Google Scholar]
- 66. Vinit N, Gueneuc A, Bessieres B, et al. Fetal cystoscopy and vesicoamniotic shunting in lower urinary tract obstruction: long‐term outcome and current technical limitations. Fetal Diagn Ther. 2020;47:74–83. [DOI] [PubMed] [Google Scholar]
- 67. Abdennadher W, Chalouhi G, Dreux S, Rosenblatt J, Favre R, Guimiot F, et al. Fetal urine biochemistry at 13‐23 weeks of gestation in lower urinary tract obstruction: criteria for in‐utero treatment. Ultrasound Obstet Gynecol. 2015;46:306–11. [DOI] [PubMed] [Google Scholar]
- 68. Kondo A, Akada S, Akiyama K, Arakawa M, Ichi S, Inamoto Y, et al. Real prevalence of neural tube defects in Japan: how many of such pregnancies have been terminated? Congenit Anom (Kyoto). 2019;59:118–24. [DOI] [PubMed] [Google Scholar]
- 69. Meuli M, Meuli‐Simmen C, Hutchins GM, Seller MJ, Harrison MR, Adzick NS. The spinal cord lesion in human fetuses with myelomeningocele: implications for fetal surgery. J Pediatr Surg. 1997;32:448–52. [DOI] [PubMed] [Google Scholar]
- 70. Meuli‐Simmen C, Meuli M, Hutchins GM, Harrison MR, Buncke HJ, Sullivan KM, et al. Fetal reconstructive surgery: experimental use of the latissimus dorsi flap to correct myelomeningocele in utero. Plast Reconstr Surg. 1995;96:1007–11. [PubMed] [Google Scholar]
- 71. Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg. 1998;28:177–80. [DOI] [PubMed] [Google Scholar]
- 72. Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–6. [DOI] [PubMed] [Google Scholar]
- 73. Bruner JP, Tulipan N, Paschall RL, Boehm FH, Walsh WF, Silva SR, et al. Fetal surgery for myelomeningocele and the incidence of shunt‐dependent hydrocephalus. JAMA. 1999;282:1819–25. [DOI] [PubMed] [Google Scholar]
- 74. Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826–31. [DOI] [PubMed] [Google Scholar]
- 75. Danzer E, Flake AW. In utero repair of myelomeningocele: rationale, initial clinical experience and a randomized controlled prospective clinical trial. Neuroembryology Aging. 2008;4:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kitano Y, Chiba T, Kuroda T, et al. Open fetal surgery for congenital cystic adenomatoid malformation complicated with fetal hydrops—a case report. J Japan Society Perinatal Neonatal Med. 2005;41:67–72. [Google Scholar]
- 77. Takahashi YO, Wada S, Miya M, Akaishi R, Sugibayashi R, Ozawa K, et al. Nationwide survey of fetal myelomeningocele in Japan: background for fetal surgery. Pediatr Int. 2019;61:715–9. [DOI] [PubMed] [Google Scholar]
- 78. Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–3. [DOI] [PubMed] [Google Scholar]
- 79. Rychik J. Hypoplastic left heart syndrome: from in‐utero diagnosis to school age. Semin Fetal Neonatal Med. 2005;10:553–66. [DOI] [PubMed] [Google Scholar]
- 80. Barron DJ, Kilby MD, Davies B, Wright JG, Jones TJ, Brawn WJ. Hypoplastic left heart syndrome. Lancet. 2009;374:551–64. [DOI] [PubMed] [Google Scholar]
- 81. Alsoufi B, Bennetts J, Verma S, Caldarone CA. New developments in the treatment of hypoplastic left heart syndrome. Pediatrics. 2007;119:109–17. [DOI] [PubMed] [Google Scholar]
- 82. Allan LD, Maxwell DJ, Carminati M, Tynan MJ. Survival after fetal aortic balloon valvoplasty. Ultrasound Obstet Gynecol. 1995;5:90–1. [DOI] [PubMed] [Google Scholar]
- 83. Kohl T, Sharland G, Allan LD, Gembruch U, Chaoui R, Lopes LM, et al. World experience of percutaneous ultrasound‐guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol. 2000;85:1230–3. [DOI] [PubMed] [Google Scholar]
- 84. Arzt W, Wertaschnigg D, Veit I, Klement F, Gitter R, Tulzer G. Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: experience and results of 24 procedures. Ultrasound Obstet Gynecol. 2011;37:689–95. [DOI] [PubMed] [Google Scholar]
- 85. Tulzer A, Arzt W, Gitter R, Sames‐Dolzer E, Kreuzer M, Mair R, et al. Valvuloplasty in 103 fetuses with critical aortic stenosis: outcome and new predictors for postnatal circulation. Ultrasound Obstet Gynecol. 2021;59:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pickard SS, Wong JB, Bucholz EM, Newburger JW, Tworetzky W, Lafranchi T, et al. Fetal aortic Valvuloplasty for evolving Hypoplastic left heart syndrome: a decision analysis. Circ Cardiovasc Qual Outcomes. 2020;13:e006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moon‐Grady AJ, Morris SA, Belfort M, Chmait R, Dangel J, Devlieger R, et al. International fetal cardiac intervention registry: a worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol. 2015;66:388–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


