FIGURE 2.
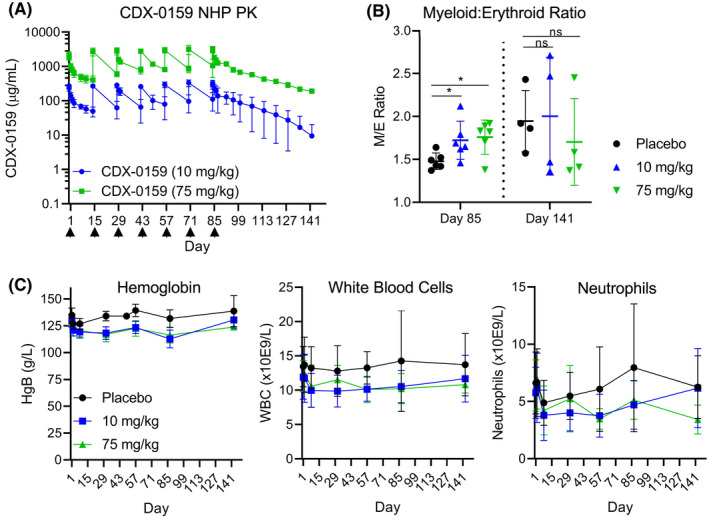
Repeat dosing of CDX‐0159 in non‐human primates does not induce significant myelosuppression. (A) CDX‐0159 administration every two weeks results in high drug levels and exposure at 10 and 75 mg/kg throughout the dosing period 13weeks and post‐treatment recovery (8 weeks). Arrows denote dosing. (B) In bone marrow smears, CDX‐0159‐induced marginal increases in myeloid/erythroid ratios at the end of treatment which were not seen at the end of the recovery period. Cohort means (n = 6 for on‐treatment and n = 4 for recovery animals) and S.E.M. values are shown. p‐values; ns: not significant; *: p < 0.05. (C) Repeat CDX‐0159 administration induces mild rapid decreases in hemoglobin values (left) without further decline despite high and prolonged drug exposure. A similar pattern is observed in total leukocyte count (center) and neutrophils (right), although the total counts exhibit greater intra‐subject variability. Cohort means (n = 10 for on‐treatment and n = 4 for recovery animals) and S.E.M. values are shown. In all cases, no meaningful differences in hematology values between genders were observed
