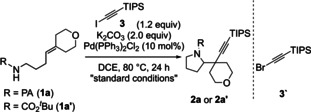
Table 1.
Exploration of reaction conditions.
|
| ||
|---|---|---|
|
|
|
|
|
Entry |
Variation from “standard condtions”[a] |
Yield [%][b] |
|
1 |
None |
85 |
|
2 |
Pd(OAc)2 |
70 |
|
3 |
Si i Pr3‐protected bromoalkyne (3′) |
<5 |
|
4 |
t BuO2C‐ instead of PA (1 a′) |
<5 |
|
5 |
1 a′, 3′ |
<5 |
|
6 |
1 a′, Pd(dba)2, DPE‐Phos, NaOtBu, PhMe |
<5 |
|
7 |
1 a′, 3′, Pd(dba)2, DPE‐Phos, NaOtBu, PhMe |
<5 |
[a] Reactions conducted using 0.1 mmol 1 a at 0.1 M. [b] Yield determined by 1H NMR analysis of unpurified reaction mixture. DPE‐Phos=Bis[(2‐diphenylphosphino)phenyl] ether.