FIG. 1.
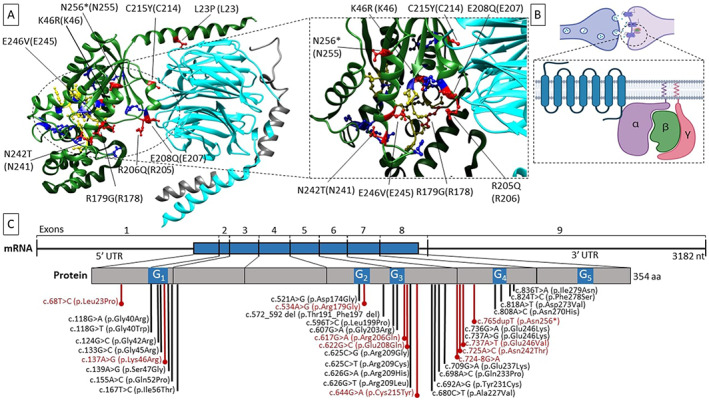
Impact of the mutations on the protein. (A) Position of the variant sites on the heterotrimeric complex containing the Gα subunit. The heterotrimer is depicted in the resting state (GDP‐bound, PDBcode 1GG2). Subunits α, β, and γ are colored in green, cyan, and gray, respectively. Affected residues in this cohort are in red, and their position is indicated both on the human Gαo1 and on rat Gαi1 (UniProtKB ID P10824, between brackets). GDP‐binding residues are colored in yellow. Previously reported GNAO1 variants are in blue. On the right, a focused view of the GDP‐binding site is shown. (B) Cartoon model of the heterotrimeric‐αβγ G‐protein coupled‐receptor on the synaptic cleft. (C) Schematic representation of the disease‐causing variants on GNAO1 transcript (NM_020988.3) and protein (UniprotKB ID P09471‐1). The amino acids impacted by the mutations identified in this work are in red, whereas previously reported variants are in black. The blue bar on the transcript indicates the translated region. The blue segments in the protein sequence indicate the G‐motifs (containing the nucleotide binding residues)—numbered from 1 to 5. Molecular graphics are realized with UCSF Chimera (http://www.rbvi.ucsf.edu/chimera), developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41‐GM103311. The cartoon has been created with BioRender.com. aa, amino acids; nt, nucleotides; UTR, untranslated region. [Color figure can be viewed at wileyonlinelibrary.com]
