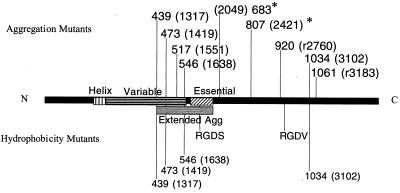
FIG. 7.
Functional domains identified in this study that disrupted aggregation (top) and hydrophobicity (bottom). All proteins generated from these insertions were expressed on the cell surface. The two mutants with increased stability at 30°C are indicated (∗). Amino acids preceding the insertions indicate the mutants (nucleotide residues are given in parentheses). The Extended Agg domain was identified by the mutations in this paper and by the previously identified aggregation functional domain (21). Note that the N-terminal aggregation domain extends into the variable region. C-terminal insertions that disrupt aggregation are hypothesized to play a structural role. Many mutants that are unable to aggregate still increase cell surface hydrophobicity.