Figure 7.
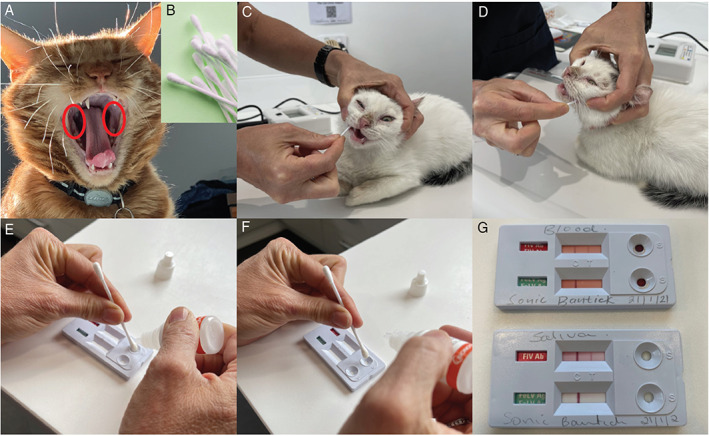
Suggested procedure for saliva testing and FIV point‐of‐care (PoC) testing. Saliva is collected from the caudal oral mucosa (A) (circled in red) using a clean cotton tip (B). The patient is held gently around the head so that the swab can be moved around freely to collect saliva (C) and (D). The saliva‐laden swab is held in the sample well of the Anigen Rapid™ FIV PoC test kit and gently blotted while double the recommended test diluent (i.e., 4 drops instead of 2 drops) is dropped onto the cotton tip of the swab (E) and (F), until the fluid begins to move across the test strip. A result is read after 10 minutes, as per the manufacturer's instructions for blood testing (G). The top strip of the test kit is for FIV antibody testing and the bottom strip is for feline leukemia virus (FeLV) antigen testing. One band in the strip indicates a negative result, while two bands in the strip indicates a positive result. In this example, the cat is FIV‐positive with both blood (top kit) and saliva (bottom kit) testing (G). None of the FIV antibody kit manufacturers (including BioNote, manufacturer of Anigen Rapid™) currently endorses using saliva instead of blood as a diagnostic specimen. PoC testing with saliva for the diagnosis of FeLV infection is not recommended. Images provided by Dr Moira van Dorsselaer.
