Fig. 2.
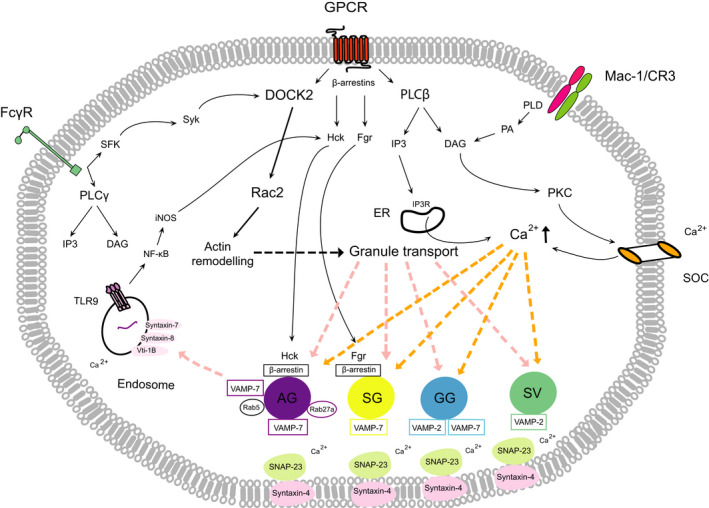
Major signaling pathways regulating neutrophil degranulation. Ligation of GPCRs, Fc receptors, or the phagocytic β2‐integrin Mac‐1/CR3 triggers degranulation through multiple, partially overlapping downstream signaling pathways, converging on activation of Rac2, and increasing intracellular calcium. Rac2 is activated through GPCR‐activated guanine nucleotide exchange factors or the SFK (Src family of tyrosine kinases)‐Syk‐DOCK2 pathway following ligation of Fcγ receptors. Calcium signaling is driven by influx of extracellular calcium via DAG/PKC‐regulated SOCs or calcium release from intracellular stores via the IP3 receptor. Chemokine binding to GPCRs leads to direct binding of β‐arrestins to the receptor, which along with the Src‐family kinases Hck or Fgr translocate to the azurophilic and SG, respectively. Activation of TLR9 in the endosome also triggers degranulation through NF‐κB/iNOS‐mediated activation of Hck. DOCK‐2‐Rac2 signaling induces actin remodeling and granule transport from the cytosol to the plasma membrane. Fusion of granules with the plasma membrane is governed by calcium‐dependent formation of SNARE complexes, consisting of granule VAMPs and cognate SNAPs/syntaxins on the plasma membrane. The Rab GTPase, Rab5, expressed on a subset of azurophilic granules, mediates their fusion with endosomes. AG, azurophilic (primary) granules; IP3R, IP3 receptor; SG, secretory (secondary) granules.
