Abstract
Background
Thrombosis may complicate autoimmune hemolytic anemia (AIHA), but its predictors are still lacking, and no clear‐cut indications for anticoagulant prophylaxis are available.
Objectives
To characterize frequency and severity of thromboses in AIHA patients and identify risk factors for thrombosis that may advise primary anticoagulant prophylaxis.
Patients/Methods
A total of 287 consecutive AIHA patients diagnosed and followed from 1978 at a tertiary Italian center were retrospectively studied; 174 of them were prospectively evaluated from January 2020 until December 2021. AIHA relapse, thrombosis occurrence, and primary anticoagulant prophylaxis were evaluated.
Results
Thirty‐three AIHA patients (11.4%) experienced thrombosis, 70% of whom hospitalized. The cumulative thrombosis incidence was higher in patients with lactate dehydrogenase (LDH) ≥ 1.5 (hazard ratio [HR] 3.22), in those experiencing infections (HR 3.57), receiving transfusions (HR 3.06), rituximab (HR 3.3), or cyclophosphamide (HR 2.67). By multivariable analysis, LDH, transfusions, rituximab, and cyclophosphamide treatment emerged as independent factors associated with thrombosis. Among 174 patients prospectively followed in the past 2 years, we observed 70 acute hemolytic episodes in 45 patients; 33/45 displayed LDH ≥1.5 × upper limit of normal, and 17 received anticoagulant prophylaxis with low molecular weight heparin for a median of 70 days (30–300). In those receiving prophylaxis no thrombotic complications occurred, whereas five thrombotic episodes were registered in the remaining 16 cases.
Conclusions
Thrombosis was observed in about 11% of AIHA patients, mainly grade 3, and associated with intravascular hemolysis, need of transfusions, multitreatment, and infections, advising primary anticoagulant prophylaxis in these settings.
Keywords: anticoagulant prophylaxis, autoimmune hemolytic anemia, intravascular hemolysis, multi‐treatment, thrombosis
Essentials.
Thromboses may complicate the clinical course of autoimmune hemolytic anemia.
Among 287 patients, 11.4% experienced thrombosis, mainly pulmonary embolism.
Predictors of thrombosis were severe hemolysis (LDH ≥ 1.5 × upper limit of normality), need of transfusion, rituximab and cyclophosphamide, and concomitant infections.
Primary thromboprophylaxis with low molecular weight heparin is advised in these settings.
1. INTRODUCTION
Autoimmune hemolytic anemia (AIHA) is a clinically heterogeneous disease ranging from mild compensated cases to highly hemolytic ones requiring transfusions, immunosuppressive therapy, and hospital admission. Disease severity depends on many pathogenic factors including autoantibody type, ability to fix and activate the complement cascade, the degree of reticuloendothelial activation, and bone marrow compensatory response. 1 , 2 To further increase disease burden, the occurrence of thrombotic complications has been described in various reports 3 , 4 , 5 , 6 , 7 and has been related to increased morbidity and mortality in AIHA patients. 5 , 6 , 8 The pathogenesis is still largely obscure and possibly includes the release of free hemoglobin (Hb) and consequent nitric oxide depletion, the proinflammatory/procoagulant autoimmune status, and the several interactions between the complement system and the coagulation cascade. 9 Moreover, AIHA may be secondary to a number of neoplastic, infectious, and other autoimmune conditions that may further increase thrombotic risk. Predictors of thrombosis in AIHA are still lacking, the routine use of thrombophilia screening is not yet recommended, 1 , 2 and no clearcut indications for anticoagulant prophylaxis are available. In this study, we aimed at characterizing the frequency and severity of thrombotic episodes in a large series of AIHA patients followed at a reference center and at individuating risk factors for thrombosis that may advocate for primary anticoagulant prophylaxis.
2. PATIENTS AND METHODS
2.1. Study design
We retrospectively evaluated the frequency of thrombotic events in 287 consecutive patients with AIHA diagnosed and followed from June 1978 at a tertiary center in Milan, Italy. Moreover, we prospectively studied 174 patients (belonging to the same initial cohort) experiencing an AIHA relapse from January 2020 until December 2021, to evaluate the occurrence of thrombosis and the use of primary anticoagulant prophylaxis. The study was conducted according to the Declaration of Helsinki and approved by the local Ethical Committee.
2.2. Clinical parameters and outcome measures
AIHA was classified according to direct antiglobulin test (DAT) as warm (either immunoglobulin G [IgG] positive, or IgG positive plus complement, C, at low titer), cold (C positive), mixed (IgG plus complement positive autoagglutination at room temperature, and high titer cold agglutinins), or atypical (DAT negative, IgA positive), as per most recent guidelines. 2 The frequency, type, and severity of thrombotic episodes, and the therapy administered were evaluated both retrospectively and in the prospective part of the study. Additionally, the association of thrombotic complications with disease characteristics (AIHA type, blood counts, hemolytic markers) at baseline and the time of thrombosis, and with the number and type of AIHA therapies required. Furthermore, the Padua score for thrombosis, that includes various established risk factors (recent cancer/chemo‐/radiotherapy in past 6 months, previous thrombosis, bedrest > 3 days, known thrombophilia, surgery/trauma, age > 70 years, cardiac/respiratory failure, myocardial infarction/stroke, obesity, ongoing hormonal treatment) was evaluated in all patients experiencing a thrombotic complication and in a group of 99 age‐ and sex‐matched AIHA patients who did not experience a thrombotic episode. Finally, the association of thrombosis with the occurrence of other AIHA‐related complications (i.e., infections, concomitant immune thrombocytopenia, namely Evans syndrome), and death, was also studied.
2.3. Statistical analysis
Mann–Whitney and χ2 tests were used for comparison of continuous and categorical variables, respectively. We performed survival/incidence analysis after truncating follow‐up time (time since diagnosis) at 12 years (because there were few patients with longer follow‐up, and only one who had a thrombosis episode). First, we plotted cumulative incidence of thrombosis (where death was treated as a competing event) by selected clinical variables. 10 Second, hazard ratios (HR) and 95% confidence intervals (CI) were calculated for the same selected variables using univariate ad multivariable Cox regression models. Variables for multivariable analysis were selected from those emerged as significant in univariate analysis. Statistical analyses were performed with Stata 17 software (StataCorp., 2021).
3. RESULTS
3.1. Frequency and characteristics of thrombotic events
In the retrospective analysis, during a median follow up of 51 months (2–538) 33 of 287 AIHA patients experienced a thrombotic complication (11.4%), 70% of whom hospitalized. Median time from AIHA diagnosis to the first thrombotic complication was 4 months (0–144), and 16 episodes (48%) occurred within the first 3 months. Most patients had been diagnosed between 2000 and 2022 (N = 243, 85%), 22 experienced thrombosis between 2010 and 2022, 10 between 2010 and 2022, and only 1 in the 1990s (Table S1). We observed mainly venous thromboses, including pulmonary embolism (PE, N = 13), followed by deep venous thrombosis (DVT) of lower limbs (N = 8), splanchnic vessels thrombosis (N = 2, one portal and one splenic vein thrombosis), superficial thrombophlebitis (N = 3), and catheter‐associated thrombosis (N = 2). Additionally, three myocardial infarctions and two strokes were also registered. Of note, five patients had multiple thrombotic episodes (two in three, three in one, and five in one). Twelve cases were started on low molecular weight heparin, 17 oral anticoagulants, four antiplatelets agents, and two died because of the event (massive PE and stroke).
At the time of thrombotic complication, none of the patients was receiving antithrombotic prophylaxis or antiplatelet agents; 17 patients (51%) were receiving active therapy for their AIHA including high‐dose steroids (N = 16), cyclophosphamide (N = 3), rituximab (N = 2), intravenous immunoglobulins (N = 2), cyclosporine (N = 1), and recombinant erythropoietin (N = 1), and two patients had been splenectomized within the past 6 months from thrombosis.
Importantly, 9/33 patients (27%) had an associated clinical condition that may have increased the thrombotic risk including six Evans syndrome (association of immune thrombocytopenia or neutropenia, not receiving thrombopoietin receptor agonists), one antiphospholipid syndrome (diagnosed after multiple DVTs), one an associated paroxysmal nocturnal hemoglobinuria (experiencing bilateral massive PE), and 1 thalasso‐drepanocytosis (the patient had 75% HbS levels, bilateral PE, and died from ischemic stroke during AIHA relapse).
3.2. Association of thrombosis with AIHA features, therapies, and complications
Table 1 shows clinical and laboratory parameters of AIHA patients with and without thrombosis: baseline Hb was significantly lower (p = .02) and LDH levels were significantly higher (p = .01) in patients experiencing thrombosis, whereas reticulocytes and bilirubin levels were comparable. At the time of thrombosis, Hb significantly ameliorated (p = .002) and LDH slightly decreased but still persisted elevated compared with baseline, likely because of treatment. Finally, other factors such as age, sex, AIHA type, white blood cells, and platelets were similar in the two groups.
TABLE 1.
Clinical and laboratory parameters of autoimmune hemolytic anemia (AIHA) patients divided according to the development of thrombotic complications
| Thrombosis (N = 33) | No thrombosis (N = 254) | |
|---|---|---|
| Median follow‐up, days (range) | 1584 (71–8915) | 950 (28–16 483) |
| Males, N (%)/females, N (%) | 13 (39)/20 (61) | 98 (38)/156 (62) |
| Median age, years (range) | 65 (24–84) | 63 (5–96) |
| Type of AIHA, N (%) | ||
| Warm IgG+ | 9 (27.3) | 102 (40) |
| Warm IgG + C | 6 (18.3) | 24 (9.5) |
| Cold agglutinin disease | 11 (33.3) | 92 (36) |
| Mixed AIHA | 3 (9.1) | 17 (7) |
| Atypical AIHA | 4 (12) | 19 (7.5) |
| Hematologic parameters at diagnosis | ||
| Median Hb, g/dL (range) | 6.5 (2–9.9) | 7.3 (2.1–14.1) * |
| Median Ret, × 10e9/L (range) | 162 (49–570) | 165 (40–780) |
| Median LDH, × ULN (range) | 2.2 (1.18–14) | 1.5 (0–18) * |
| Median unconjugated bilirubin, mg/dL (range) | 1.38 (0.28–6) | 1.1 (0.11–6.7) |
| Median WBC, × 109/L (range) | 5.8 (3.3–14) | 7.18 (3.4–28) |
| Median PLT, × 109/L (range) | 200 (48–414) | 264 (2.66–464) |
| Median C3, mg/dL (range) | 104 (79–123) | 91 (48–116) |
| Median C4, mg/dL (range) | 17 (1–55) | 13 (6–30) |
| Hematologic parameters at thrombosis | ||
| Median Hb, g/dL (range) | 9.1 (3.2–14) | – |
| Median Ret, × 10e9/L (range) | 128 (22–400) | – |
| Median LDH, × ULN (range) | 1.49 (0–19) | – |
| Median unconjugated bilirubin, mg/dL (range) | 1.3 (0.2–4.9) | – |
| Median WBC, × 10e9/L (range) | 7.4 (1.5–25) | – |
| Median PLT, × 10e9/L (range) | 211 (77–794) | – |
Abbreviations: C3 and C4, complement fraction 3 and 4; Hb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelets; Ret, reticulocytes; WBC, white blood cells.
p < 0.05.
Regarding AIHA therapies (Table 2), patients experiencing thrombosis had received more frequently two, three, or four lines (p = .001, p = .0001, p = .01, respectively). Considering specific treatments, AIHA patients with thrombotic complications had been more frequently transfused (70 vs 41%, p = .005), and treated with rituximab (76 vs 34%, p < .0001), cytotoxic immunosuppressors (45 vs 19%, p = .0001), recombinant erythropoietin (21 vs 7%, p = .04), and bortezomib (9 vs 1.5%, p = 0.03). Additionally, thrombotic episodes were more common among splenectomized patients (18% vs 8%), although not significantly. Among complications, infections were the only associated with thrombosis occurrence (36 vs 14%, p = .05).
TABLE 2.
Therapies and complications in patients with autoimmune hemolytic anemia (AIHA) with or without thrombosis
| Thrombosis (N = 33) | No thrombosis (N = 254) | |
|---|---|---|
| No. of therapy lines, median (range) | 3 (1–5) | 1 (0–5)* |
| First line, N (%) | 33 (100) | 236 (93) |
| Second line, N (%) | 26 (79) | 112 (44)** |
| Third line, N (%) | 15 (45) | 33 (13)** |
| Fourth or > line, N (%) | 5 (15) | 10 (4)^ |
| Transfusions, N (%) | 23 (70) | 104 (41)* |
| Steroids, N (%) | 31 (94) | 216 (85) |
| IVIG, N (%) | 6 (18) | 34 (13) |
| Rituximab, N (%) | 25 (76) | 86 (34)** |
| Splenectomy, N (%) | 6 (18) | 20 (8) |
| Immunosuppressors, N (%) | 15 (45) | 49 (19)** |
| Erythropoietin, N (%) | 7 (21) | 19 (7)^ |
| Bortezomib, N (%) | 3 (9) | 4 (1.5)^ |
| Plasma exchange, N (%) | 1 (3) | 3 (1) |
| Complications and outcome | ||
| Acute renal failure, N (%) | 2 (6) | 6 (2) |
| Evans' syndrome, N (%) | 6 (18) | 21 (8) |
| Infections, N (%) | 12 (36) | 36 (14)* |
| Death, N (%) | 4 (12) | 46 (18) |
| Death AIHA‐related, N (%) | 3 (9) | 8 (3) |
Note: All therapies and complications have been included and not only those ongoing at the time of thrombosis. Immunosuppressors include cyclophosphamide, azathioprine, and cyclosporine A. IVIG, intravenous immunoglobulins. ^p < .05; *p ≤ .005; **p ≤ .001.
Notably, hematologic values, AIHA therapies, and complications did not differ among the various decades of diagnosis (Table S1).
3.3. Analysis of the cumulative incidence of thrombosis
The cumulative incidence of thrombosis was of 31% and was significantly higher in patients with increased LDH [hazard ratio, HR 3.22, 95% CI, 1.39–7.47, p = .009] and in those receiving transfusions [HR 3.06, 95% CI 1.46–6.4, p = .004]. Additional risk factors for thrombosis included treatment with rituximab [HR 3.3, 95% CI 1.56–7.0, p = .001], cyclophosphamide [HR 2.67, 95% CI 1.26–5.6, p = .004], and infectious complications [HR 3.57, 95% CI 1.7–7.4, p = .001] (Figure 1). Furthermore, by multivariable analysis, LDH, transfusions, rituximab, and cyclophosphamide treatment emerged as independent factors associated with thrombosis (Table S1).
FIGURE 1.
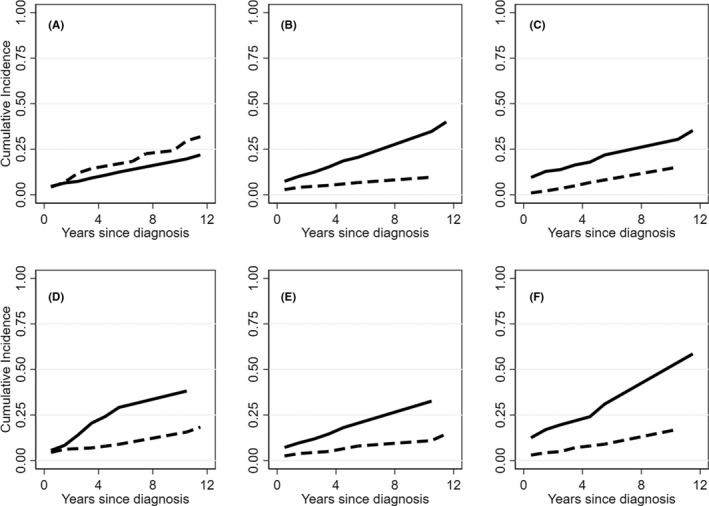
Cumulative incidence of thrombosis in autoimmune hemolytic anemia (AIHA) patients. (A) Cumulative incidence of thrombosis (continuous line) and of mortality (dashed line). (B–D) Cumulative incidence of thrombosis in panel B. Patients treated with rituximab (continuous line) or not (dashed line); panel C. Patients with LDH ≥1.5× upper limit of normality, ULN (continuous line) or not (dashed line); panel D. Patients treated with cyclophosphamide (continuous line) or not (dashed line); panel E. Patients who underwent blood transfusions (continuous line) or did not (dashed line); panel F. Patients experiencing infections (continuous line) or not (dashed line).
3.4. Other risk factors for thrombosis
As shown in Table 3, the Padua score highlighted bedrest with reduced mobility and obesity as factors significantly associated with thrombosis occurrence (39 vs. 4%, p = .00002, and 18 vs. 2%, p = .003, respectively), whereas older age, underlying neoplasms, recent traumas or surgery, heart or respiratory failure, hormonal therapy, active infection, or rheumatologic disorder were equally distributed. The total score was not different among patients with or without thrombosis. Finally, the presence of antiphospholipid antibodies was comparable in the two groups (lupus anticoagulant 12% vs. 24%, anticardiolipin antibodies 12% vs. 8%, anti‐beta2‐glycoprotein1 antibodies 9% vs. 8%), although they had been tested in a minority of patients (N = 58, 15 with a complete thrombophilia screening).
TABLE 3.
Padua score in autoimmune hemolytic anemia (AIHA) with or without thrombosis
| Padua score in patients and age and sex matched controls | Thrombosis (N = 33) | No thrombosis (N = 99)* |
|---|---|---|
| Active cancer, N (%) | 5 (15) | 10 (10) |
| Previous VTE, N (%) | 0 | 0 |
| Reduced mobility, N (%) | 13 (39) | 4 (4)** |
| Already known thrombophilic condition, N (%) | 4 (12) | 0 |
| Recent trauma/surgery (≤1 month), N (%) | 3 (9) | 8 (8) |
| Age ≥ 70 years, N (%) | 13 (39) | 28 (28) |
| Heart/respiratory failure, N (%) | 4 (12) | 0 |
| Acute MI and/or ischemic stroke, N (%) | 0 | 0 |
| Acute infection/rheumatologic disorder, N (%) | 4 (12) | 17 (17) |
| Obesity (BMI ≥30), N (%) | 6 (18) | 2 (2)*** |
| Ongoing hormonal therapy, N (%) | 0 | 1 (1) |
| Total score, median (range) | 1 (0–3) | 1 (0–5) |
Note: *Sex and age matched AIHA patients who had not experienced thrombosis. **p = .00002; ***p = .003.
Abbreviations: BMI, body mass index; VTE, venous thromboembolism.
3.5. 2‐year prospective follow up
In the past 2 years (January 2020–December 2021), among 174 patients prospectively followed up, we observed 70 acute hemolytic episodes in 45 patients (26 warm IgG, six warm IgG + C, 11 CAD, and two DAT negative cases), including 16 new diagnoses (13 warm IgG, two warm IgG + C, and one CAD) and 29 relapses. Eight patients were already on direct oral anticoagulants because of previous thrombotic events or atrial fibrillation, and none experienced thrombosis. Notably, 33/45 patients displayed LDH ≥1.5 × upper limit of normal (ULN), and 17 received anticoagulant prophylaxis with low molecular weight heparin 100 U/kg per day (i.e., 4000–8000 U per day), as per physician choice, until LDH normalization (median time of 70 days, range 30–300 days). In those receiving prophylaxis no thrombotic complications occurred, whereas five thrombotic episodes were registered in the remaining 16 cases (all PE, p = .01 vs patients receiving anticoagulants). These thrombotic episodes occurred at a median of 20 days (2–90) from initial diagnosis and patients had median Hb levels of 10.3 g/dL (8.5–2.6) and LDH values of 1.6 × ULN (1.5–10) (Table S1).
4. DISCUSSION
Here we report that about 10% of AIHA experience thrombotic events, more frequently involving the venous district, and mainly pulmonary embolism. The prevalence observed was slightly lower than that described in other retrospective studies, which, however, included different AIHA populations (ie, CAD or wAIHA) and were mainly based on data extracted from electronic records. 3 , 4 , 8 More in details, recent epidemiologic studies found a frequency of about 20% of venous and arterial thrombosis (including ischemic stroke) in CAD and wAIHA, 3 , 4 , 8 , 11 , 12 significantly higher than in the general population. 4 , 8 , 11 The calculated pooled risk ratio of thrombosis was 2.63 (95% CI, 1.37–5.05) in a systematic review, but only four studies (three cohort studies and one cross‐sectional study) not designed to specifically assess thrombosis in AIHA were included. 13 , 14 , 15 , 16 , 17 The added value of our study is to present the largest series of AIHA consecutive patients with detailed clinical data, specifically evaluated to assess predictors of thrombosis. We found that thrombosis occurrence was associated with hyper‐hemolysis (LDH ≥ 1.5 × ULN) and multitreatment, as AIHA associated factors. In particular, the need of transfusions, rituximab, and cyclophosphamide emerged as independent factors in multivariable analysis. The latter are generally administered in more severe cases, suggesting that thrombotic risk mainly associates with disease severity rather than with a specific treatment. Among AIHA complications, infections were also independently associated with thrombosis in our cohort, highlighting the contribution of the septic state to thrombo‐inflammation. 18 Other predisposing conditions included obesity and bedrest; the latter being related to hospitalization because of severe AIHA. The presence of antiphospholipid antibodies did not emerge as a significant risk factor, although the limited number of tested cases did not allow definite conclusions. The pivotal role of the hyper‐hemolytic process is strengthened by the prospective analysis where we observed thrombotic events in 30% of patients with LDH ≥1.5 × ULN who did not receive anticoagulant prophylaxis. Contrarily, no thromboses were observed in those receiving prophylactic heparin. Interestingly, half of all episodes occurred within the first 3 months from AIHA onset in the retrospective analysis, and within 20 days from the hemolytic flare in the prospective part of the study. This is in line with what reported in the French wAIHA study where most episodes occurred within the first weeks from diagnosis, 3 and for ischemic strokes, that occurred within the first year in 57% of cases, 8 again indicating the contribution of active disease to the thrombotic process. Accordingly, we found that at the time of thrombosis Hb values were greater compared with AIHA onset and associated with persistently elevated LDH levels. Thus, it may be hypothesized that augmented red cell mass and nitric oxide depletion during intravascular hemolysis both contribute to the thrombotic process. Additionally, a recent study demonstrated an increased level of erythrocyte derived microparticles in hyper‐hemolytic AIHA, particularly after splenectomy. 19 Such microparticles are enriched in procoagulant mediators and phosphatidyl serine and may further favor thrombotic episodes. 20 Although splenectomy is a recognized risk factor for thrombosis in various conditions, 21 , 22 , 23 , 24 , 25 here we found only a slightly higher frequency of thrombosis in splenectomized patients. This may be due to the low number and progressive decrease of splenectomy over time, and to the higher awareness of splenectomy‐related thrombotic risk. 2 , 23 Finally, we found that one third of patients experiencing thrombosis had an associated disease, namely Evans' syndrome, paroxysmal nocturnal hemoglobinuria, and hemoglobinopathy. Although rare, these conditions should be considered because they may greatly increase thrombotic risk and be potentially fatal. 26 , 27 , 28 , 29 , 30
Our study carries several limitations, including the retrospective nature, the long span of observation, and the lack of systematic evaluation of thrombophilia screening. However, the prospective part of the study strengthened the message to administer primary anticoagulant prophylaxis in patients with AIHA and active hemolysis (LDH ≥ 1.5 × ULN). This should be further advised in severe hospitalized cases requiring transfusions, therapy with rituximab or cyclophosphamide, and in those experiencing infectious complications. The impact of additional non AIHA‐related predisposing conditions should be considered as per recent consensus 2 , 26 and evaluated on a case‐by‐case basis. The duration of anticoagulant prophylaxis is still a matter of debate, and although most episodes happened within the first weeks, our results showed the occurrence of late‐onset thrombotic events, advising to continue heparin until LDH normalization. The results of an ongoing randomized prospective trial evaluating long‐term anticoagulant prophylaxis with apixaban in AIHA patients will possibly clarify this issue (NCT05089227). 31
5. CONCLUSIONS
Thrombotic episodes occurred in 11% of AIHA patients, were mainly grade 3, and required hospitalization in the great majority of cases. Intravascular hemolysis, need of transfusions, therapy with rituximab and cyclophosphamide, and infections emerged as the main risk factors, advising primary anticoagulant prophylaxis in these settings.
AUTHOR CONTRIBUTIONS
B.F., M.B., J.A.G., and W.B. followed patients, collected data, and wrote the manuscript. A.Z. collected data. D.C. performed statistical analysis. All authors revised the manuscript for important intellectual content.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest to disclose.
ETHICAL APPROVAL
Ethics approval and consent to participate were obtained for this study.
CONSENT FOR PUBLICATION
All authors approved present submission.
Supporting information
Table S1
Table S2
Table S3
Fattizzo B, Bortolotti M, Giannotta JA, Zaninoni A, Consonni D, Barcellini W. Intravascular hemolysis and multitreatment predict thrombosis in patients with autoimmune hemolytic anemia. J Thromb Haemost. 2022;20:1852‐1858. doi: 10.1111/jth.15757
Manuscript handled by: Riitta Lassila
Final decision: Riitta Lassila, 09 May 2022
Funding information
No funding sources to declare.
DATA AVAILABILITY STATEMENT
All data are available within the manuscript and further may be available upon reasonable request to the corresponding author.
REFERENCES
- 1. Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385(15):1407‐1419. [DOI] [PubMed] [Google Scholar]
- 2. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41:100648. [DOI] [PubMed] [Google Scholar]
- 3. Audia S, Bach B, Samson M, et al. Venous thromboembolic events during warm autoimmune hemolytic anemia. PLoS One. 2018;13(11):e0207218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broome CM, Cunningham JM, Mullins M, et al. Increased risk of thrombotic events in cold agglutinin disease: a 10‐year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930‐2936. [DOI] [PubMed] [Google Scholar]
- 6. Barcellini W, Zaninoni A, Fattizzo B, et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hematol. 2018;93(9):E243‐E246. [DOI] [PubMed] [Google Scholar]
- 7. Berentsen S, Barcellini W, D'Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136(4):480‐488. [DOI] [PubMed] [Google Scholar]
- 8. Lund Hansen D, Maquet J, Lafaurie M, Berentsen S, Frederiksen H, Moulis G & Gaist D Increased risk of ischemic stroke amongst patients with primary autoimmune hemolytic anemia in Denmark and France. Abstract 849. Annual Meeting of the American Society of Hematology, Atlanta, December 2021.
- 9. Barcellini W, Zaninoni A, Giannotta JA, Fattizzo B. New insights in autoimmune hemolytic anemia: from pathogenesis to therapy stage 1. J Clin Med. 2020;9(12):3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bylsma LC, Gulbech Ording A, Rosenthal A, et al. Occurrence, thromboembolic risk, and mortality in Danish patients with cold agglutinin disease. Blood Adv. 2019;3(20):2980‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schär DT, Daskalakis M, Mansouri B, Rovo A, Zeerleder S. Thromboembolic complications in autoimmune hemolytic anemia: retrospective study. Eur J Haematol. 2022;108(1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ungprasert P, Tanratana P, Srivali N. Autoimmune hemolytic anemia and venous thromboembolism: a systematic review and meta‐analysis. Thromb Res. 2015;136(5):1013‐1017. [DOI] [PubMed] [Google Scholar]
- 14. Sokol RJ, Hewitt S, Stamp BK. Autoimmune haemolysis: an 18‐year study of 865 cases referred to a regional transfusion centre. Br Med J. 1981;282:2023‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single‐center experience with 60 patients. Am J Hematol. 2014;89(9):E150‐E155. [DOI] [PubMed] [Google Scholar]
- 16. Allgood JW, JrH C. Idiopathic acquired autoimmune hemolytic anemia a review of forty‐seven cases treated from 1955 through 1965. Am J Med. 1967;43:254‐273. [DOI] [PubMed] [Google Scholar]
- 17. Lecouffe‐Desprets M, Néel A, Graveleau J, et al. Venous thromboembolism related to warm autoimmune hemolytic anemia: a case‐control study. Autoimmun Rev. 2015;14(11):1023‐1028. [DOI] [PubMed] [Google Scholar]
- 18. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barcellini W, Zaninoni A, Giannotta JA, et al. Circulating extracellular vesicles and cytokines in congenital and acquired hemolytic anemias. Am J Hematol. 2021;96(4):E129‐E132. [DOI] [PubMed] [Google Scholar]
- 20. Devalet B, Mullier F, Chatelain B, Dogné JM, Chatelain C. The central role of extracellular vesicles in the mechanisms of thrombosis in paroxysmal nocturnal haemoglobinuria: a review. J Extracell Vesicles. 2014;24:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lafaurie M, Maquet J, Baricault B, et al. Risk factors of hospitalisation for thrombosis in adults with primary immune thrombocytopenia, including disease‐specific treatments: a French nationwide cohort study. Br J Haematol. 2021;195(3):456‐465. [DOI] [PubMed] [Google Scholar]
- 22. Capecchi M, Ciavarella A, Artoni A, Abbattista M, Martinelli I. Thrombotic complications in patients with immune‐mediated hemolysis. J Clin Med. 2021;10(8):1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho G, Brunson A, Keegan THM, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with autoimmune hemolytic anemia. Blood Cells Mol Dis. 2020;81:102388. [DOI] [PubMed] [Google Scholar]
- 24. Al Barhi T, Wali Y, Al Sibai S, Al BZ. Extensive porto‐splenic venous thrombosis postsplenectomy in a sickle cell disease: a rare complication. BMJ Case Rep. 2022;15(1):e245085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyle S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121(23):4782‐4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fattizzo B, Michel M, Giannotta JA, et al. Evans syndrome in adults: an observational multicenter study. Blood Adv. 2021;5(24):5468‐5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fattizzo B, Giannotta J, Zaninoni A, Kulasekararaj A, Cro L, Barcellini W. Small paroxysmal nocturnal hemoglobinuria clones in autoimmune hemolytic anemia: clinical implications and different cytokine patterns in positive and negative patients. Front Immunol. 2020;11:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lizarralde‐Iragorri MA, Shet AS. Sickle cell disease: a paradigm for venous thrombosis pathophysiology. Int J Mol Sci. 2020;21(15):5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2021;137(10):1304‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delvasto‐Nuñez L, Jongerius I, Zeerleder S. It takes two to thrombosis: hemolysis and complement. Blood Rev. 2021;50:100834. [DOI] [PubMed] [Google Scholar]
- 31.Efficacy of Prolonged Anticoagulation for Primary Prevention of Venous Thromboembolic Disease in Autoimmune Hemolytic Anemia: a Prospective, Phase II, Randomized, Multicenter Study (API‐AHAI). Accessed February 24, 2022. https://clinicaltrials.gov/ct2/show/NCT05089227
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
All data are available within the manuscript and further may be available upon reasonable request to the corresponding author.


