FIGURE 3.
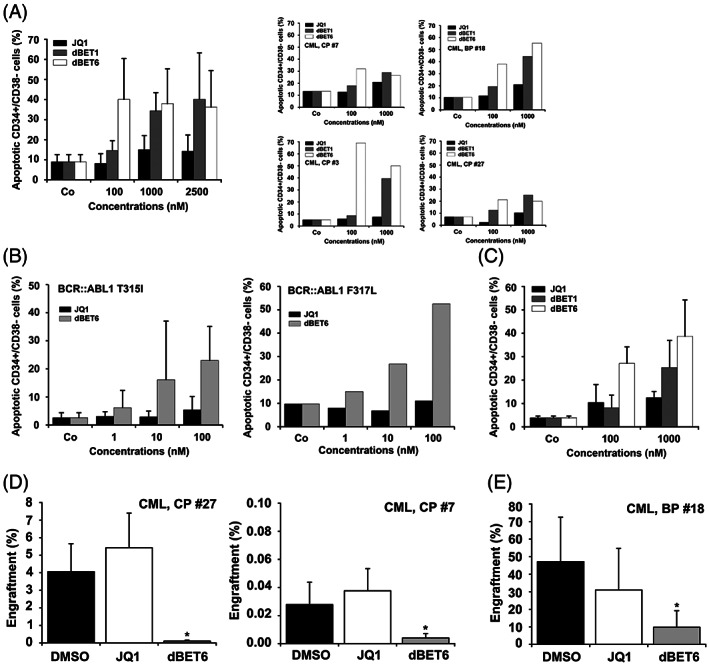
Effects of JQ1, dBET1 and dBET6 on survival and engraftment of CML LSC. (A) Primary CML MNC from three patients with CML CP and one with CML BP were incubated with various concentrations of JQ1, dBET1, or dBET6 at 37°C for 48 h. LSC were defined as CD34+/CD38− cells and the percentage of Annexin V+ (apoptotic) cells were analyzed among DAPI‐negative cells by flow cytometry. Results represent the mean ± SD from four independent experiments (left panel). The right panels show single experiments in individual CML samples. (B) Primary CML MNC from three patients with BCR::ABL1 T315I+ CML (left panel) and one with BCR::ABL1 F317L+ CML (right panel) were incubated with various concentrations of JQ1 or dBET6 for 48 h. Then, the percentage of Annexin V+ LSC (CD34+/CD38−) among DAPI‐negative cells (apoptotic LSC) were analyzed by flow cytometry. Results in the left panel represent the mean ± SD from three independent experiments. (C) Normal BM MNC were incubated with various concentrations of JQ1, dBET1, or dBET6 at 37°C for 48 h. Thereafter, normal stem cells were defined as CD34+/CD38− cells and the percentage of Annexin V positive cells (apoptotic cells) were analyzed among DAPI‐negative cells by flow cytometry. Results represent the mean ± SD from three independent experiments. (D) CD34+ CML CP cells were incubated in medium with DMSO (0.01%), 1 μM JQ1 or 1 μM dBET6 at 37°C for 4 h. Thereafter cells were harvested, washed and injected i.v. into NSGSCF mice. After a maximum period of 6 months mice were sacrificed. BM was flushed and engraftment of human myeloid CD45+/CD33+/CD19− cells determined by flow cytometry. Results are expressed as percentage of human engrafted myeloid cells and represent the mean ± SD from 4 to 5 mice per group. Asterisk: p < .05 compared to DMSO. E: T‐cell‐depleted CML BP MNC were incubated in medium containing DMSO (0.01%), 1 μM JQ1 or 1 μM dBET6 for 4 h. Then, cells were harvested, washed and injected i.v. into NSG mice. After 10 weeks mice were sacrificed. BM was flushed and engraftment of human CD45+ cells determined by flow cytometry. Results are expressed as percentage of human engrafted cells (CD45+) and represent the mean ± SD from 3 to 5 mice per group. Asterisk: p < .05 compared to DMSO.
