FIGURE 1.
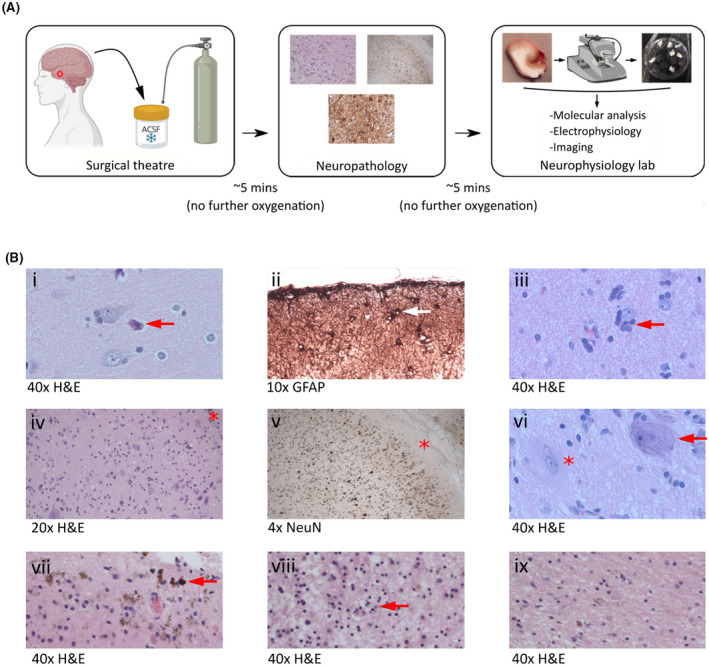
Integration of brain tissue collection for neurophysiology into the clinical workflow. (A) Resected human brain tissue specimens were obtained directly from the neurosurgical theater and immediately submerged into oxygenated ice cold artificial cerebrospinal fluid (ACSF). Specimens were transported in ACSF to the neuropathology laboratory as part of the clinical pathway. A proportion of the specimen was then returned to ACSF and taken to the research laboratory for study. (B) Examples of neuropathological observations made in specimens transported in ACSF illustrate the compatibility of our approach with the clinical pathway: (i) normal cortex and neuronal morphology with perioperative "dark cell change" (arrow); (ii) subpial gliosis (arrow); (iii) oligodendroglial hyperplasia; (iv–vi) focal cortical dysplasia type 2B characterized by disorganized lamination and dysmorphic neurons (arrow in vi; asterisk at Layer I in iv and v) and balloon cells (asterisk in vi); (vii, viii) remote hemorrhage and gliosis; (ix) a directly comparable section exhibiting remote hemorrhage and gliosis in a specimen handled in a standard way without direct ACSF exposure, indicating no qualitative impact of ACSF transport. GFAP, glial fibrillary acidic protein; H&E, hematoxylin and eosin; NeuN, neuron‐specific nuclear protein.
