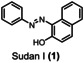
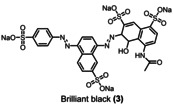
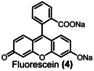
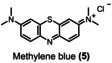
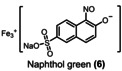
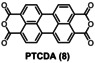
Table 1.
Electrochemical degradation of dyes in para‐periodate platform oxidizer process.[a]
|
| |||
|---|---|---|---|
|
Dye (0.1 mm) |
Degradation [kilo equiv. of Na3H2IO6] |
||
|
>90 % |
>95 % |
>99 % |
|
|
|
2.4 |
3.2 |
5.0 |
|
|
3.7 |
4.8 |
7.4 |
|
|
3.0 |
4.0 |
6.2 |
|
|
1.6 |
2.0 |
3.1 |
|
|
26.2 |
34.1 |
52.4 |
|
|
2.9 |
3.7 |
5.8 |
|
|
the data was not fit by the exponential equation; formation of intermediate species (see Supporting Information) |
||
|
|
the data was not fit by the exponential equation; formation of intermediate species (see Supporting Information) |
||
[a] reaction conditions: Flow electrolyzer (BDD|Nafion™ membrane|stainless steel, 4×12 cm2=48 cm2); dye (0.1 mm), NaOH (4 M), Na3H2IO6 (0.21 m), H2O (200 mL), j=312 mA cm−2, Q=6–149 F, rt. Degradation determined by UV‐Vis analysis. All the kilo equiv. of periodate were calculated as showed in the section 11 (Supporting Information).