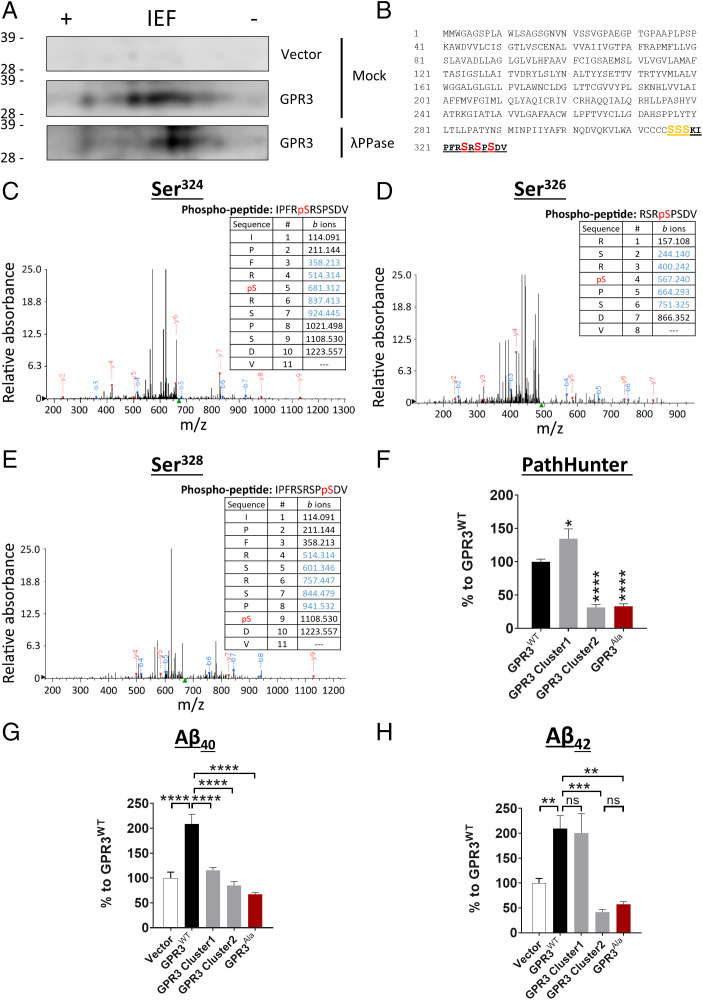
Fig. 1.
The phosphorylation status of the GPR3 C terminus dictates βarr2 recruitment and Aβ generation. (A) Cell lysates from HEK293 cells, overexpressing empty vector or HA-tagged human GPR3, were immunoprecipitated with an HA antibody and subjected to mock or λ phosphatase (λPPase) treatment. Two-dimensional electrophoresis analysis with an isoelectric focusing (IEF) strip and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (indicates that GPR3 is phosphorylated on C-terminal serine residues. (B) Schematic representation of the protein sequence of human GPR3 indicates the potential C-terminal phosphorylation sites. The peptide sequence identified by mass spectrometry analysis is highlighted in bold and underlined. The consistently identified phosphorylated residues Ser324, Ser326, and Ser328 are highlighted in red. The putative phosphorylated residues Ser316, Ser317, and Ser318 are highlighted in orange. n = 3 independent experiments. (C–E) Representative mass spectra and fragmentation tables show the three phosphorylated residues; detected b and y ions are indicated in blue and red, respectively. (F–H) Alanine mutagenesis of both cluster 1 and 2 serine residues shows a robust reduction in βarr2 recruitment to GPR3 (F) and Aβ levels (G and H). Vector condition refers to cells transfected with an empty control plasmid without GPR3. (F-H) P < 0.0001 by one-way ANOVA. Data are presented as mean ± SEM. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA with Tukey’s post hoc test.