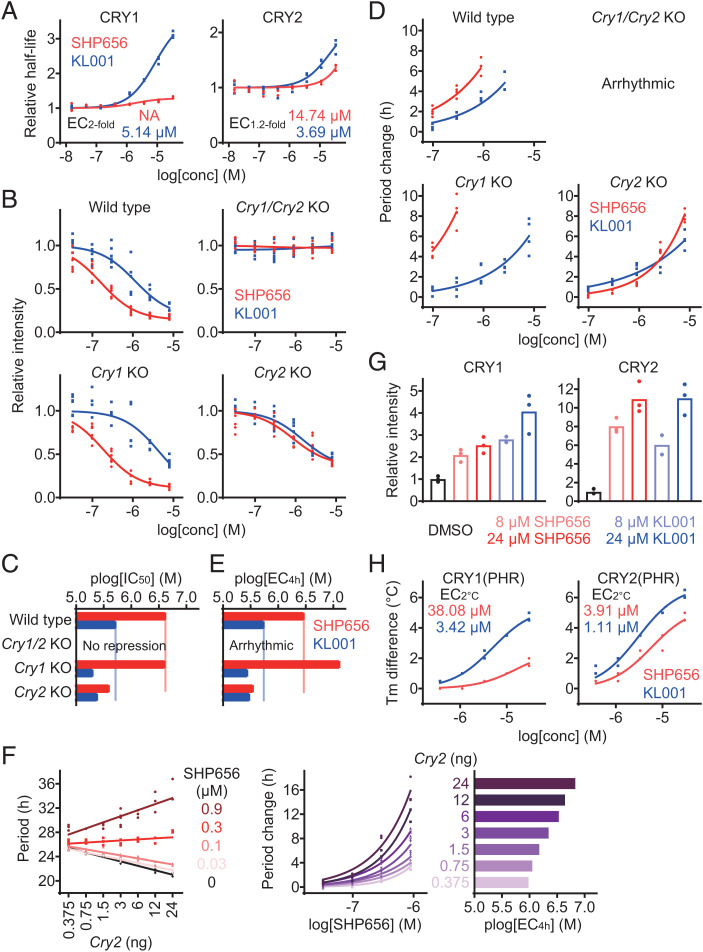
Fig. 2.
SHP656 shows selectivity against CRY2. (A) Effects of SHP656 and KL001 on CRY degradation in HEK293 cells. The half-lives of CRY1-luciferase fusion protein CRY1-LUC and CRY2-LUC relative to LUC are plotted by setting a DMSO control to one (n = 3 biologically independent samples). Concentrations for twofold (EC2-fold for CRY1) or 1.2-fold (EC1.2-fold for CRY2) stabilization are indicated. (B and C) Effects on Per2::Luc knock-in reporter activity in wild-type (Top Left, B), Cry1/Cry2 double-knockout (Top Right), Cry1 knockout (Bottom Left), and Cry2 knockout (Bottom Right) fibroblasts. Changes in luminescence intensity compared to a DMSO control are shown (B, n = 6 biologically independent samples). Concentrations for 50% inhibition (plog[IC50] that represents −log[IC50]) are plotted in C. (D and E) Effects on circadian period in Per2::Luc knock-in wild-type (Top Left, D), Cry1/Cry2 double-knockout (Top Right), Cry1 knockout (Bottom Left), and Cry2 knockout (Bottom Right) fibroblasts. Changes in period compared to a DMSO control are shown (D, n = 4 biologically independent samples). When arrhythmic, period is not plotted. Concentrations for 4-h period lengthening (plog[EC4h] that represents −log[EC4h]) are shown in E. (F) Effect of SHP656 on cellular circadian period of Bmal1-Eluc reporter rhythms in Cry1/Cry2 double-knockout fibroblasts rescued with 3 ng of Cry1 plasmid together with different amounts (0.375 ng to 24 ng) of Cry2 plasmid (n = 3 to 6 biologically independent samples). Circadian period is plotted in Left. Changes in period compared to a DMSO control of each Cry2 amount are shown in Middle. Concentrations for 4-h period lengthening (plog[EC4h]) are plotted in Right. (G) Interaction with CRY proteins in HEK293T cells. The band intensities of Flag-tagged CRY1 and HA-tagged CRY2 proteins protected from thermal denaturation in cells are plotted by setting a DMSO control to one (mean of n = 3 biologically independent samples). Compound interaction induced thermal stabilization. (H) Interaction with CRY1(PHR) and CRY2(PHR) in vitro. Changes in denaturing temperatures of recombinant CRY(PHR) proteins in the presence of various concentrations of compounds compared to a DMSO control are shown (n = 3 biologically independent samples). Concentrations for 2 °C stabilization (EC2 °C) are indicated.