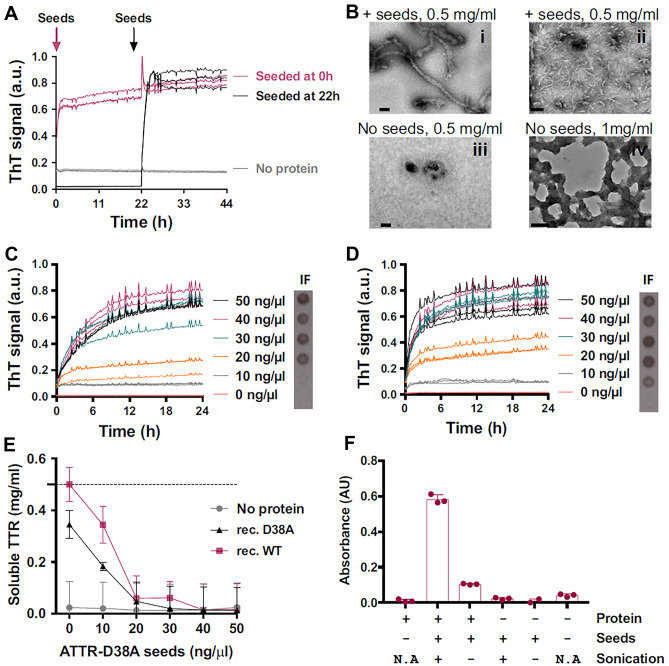
Fig. 3.
Effects of amyloid transthyretin (ATTR) seeds in vitro. Amyloid seeding at pH 4.3 of wild-type TTR by ex vivo ATTR seeds extracted from the explanted heart of an ATTR-D38A patient. (A) Amyloid seeding assay of recombinant wild-type TTR when 30 ng/μL ATTR-D38A ex vivo seeds were added at 0 h and after 22 h of preincubation, as monitored by thioflavin T (ThT) fluorescence. (B) Electron micrograph of aggregates of wild-type TTR after 24 h (i and iii) or 4 days (ii and iv) of incubation. ATTR seeds were added at 0 h (i and ii). (C and D) Amyloid seeding assays monitored by ThT fluorescence. Increasing amounts of ATTR-D38A ex vivo seeds were added at time 0 to recombinant wild-type TTR (C) or D38A TTR (D). (E) Protein concentration in the soluble fraction extracted from (C and D). Increasing concentrations of D38A seeds promote less soluble TTR due to TTR dissociation. The dashed line marks the initial protein concentration (0.5 mg/mL). (F) The 280-nm absorbance of insoluble fractions collected from an amyloid seeding assay of recombinant wild-type TTR with and without 30 ng/L sonicated or non-sonicated seeds. AU, absorbance units; a.u., arbitrary units; N/A, not applicable. Reprinted with permission from: Saelices et al. [5]