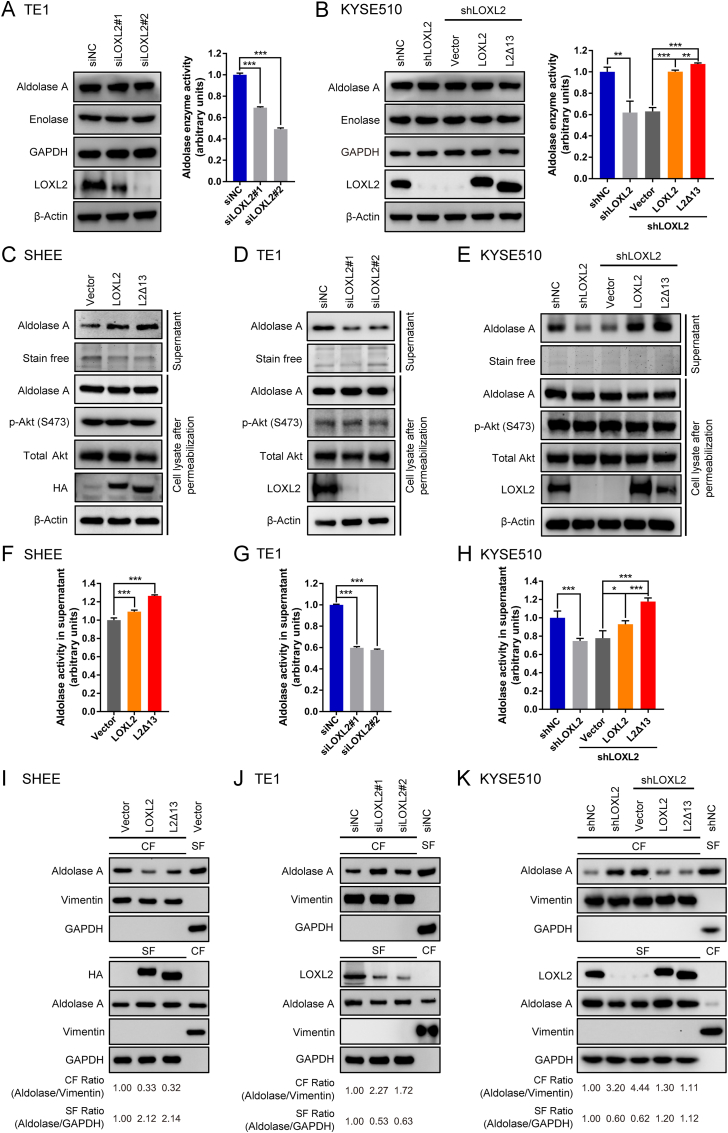
Fig. 4.
LOXL2 and L2Δ13 promote mobilization of aldolase A and increase its catalytic activity.
(A and B) Western blotting detection (left) and aldolase enzyme activity assay (right) of esophageal cancer TE1 and KYSE510 cells upon LOXL2 silencing or re-expression of either LOXL2 or L2Δ13. (C–E) Nonmalignant esophageal epithelial SHEE cells and esophageal cancer cells (TE1 and KYSE510) were permeabilized with digitonin (30 μg/mL) for 5 min. Supernatant (top two panels) and cell lysate (bottom five panels) for each assay were subjected to western blotting as indicated. (F–H) Quantification of aldolase activity in the diffusible fraction (supernatant) by immunoblotting of cells in (C–E). Bar graphs represent means ± SD, n = 3 or 4. *P < 0.05, **P < 0.01 or ***P < 0.001 by t-test. (I–K) SHEE, TE1 and KYSE510 cells were lysed and fractionated into cytoskeletal fraction (CF) and soluble fraction (SF). Fractions from indicated cells transfected with empty vector, scrambled siRNA or shRNA are controls for the fractionation procedure. Vimentin served as a marker for the CF and GAPDH for the SF.