Abstract
Purpose
Coronavirus disease 2019 (COVID-19) is a new pandemic affecting the respiratory system and caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In addition to the increased use of antibiotics, the length of stay of hospitalized patients affects the risk of bacterial infections among the COVID-19 patients. However, this pandemic has interrupted antibiotic surveillance activity and led to an information gap about the prevalence and characteristics of bacterial infection. This study aims to describe the antibiotic resistance in COVID-19 patients with culture-proven bacterial infection using a laboratory-based surveillance approach.
Patients and Methods
A retrospective study with a cross-sectional design was conducted on adult patients that confirmed positive for COVID-19 according to the International Classification of Diseases 10th Revision (ICD-10). From March 2020 to October 2021, data were obtained from the hospital information system and merged with the culture and antibiotic susceptibility test from laboratory information system at Hasan Sadikin General Hospital. The outcome is the prevalence percentage of resistance to selected antibiotics in patients with COVID-19. The resistance percentage is considered high when equal to or more than 20%.
Results
There was 2786 adult patient confirmed for COVID-19 according to the ICD-10, and 26.3% (n = 733) of them submitted clinical specimen for culture. The prevalence of bacterial infection among COVID-19 patients was 16.4%, predominating Gram-negative bacteria (GNB). The respiratory specimen dominated the positive growth culture. The GNB were predominantly discovered among the respiratory and non-respiratory specimens. High range resistance to ampicillin-sulbactam (24–100%), ceftriaxone (22–81%), cefotaxime (22–73%) and ciprofloxacin (20–86%) are observed among the GNB.
Conclusion
There is high resistance to fluoroquinolone and cephalosporins in identified isolate, commonly used as the first-line empirical treatment for respiratory and non-respiratory infection in Indonesia. The continuous antibiotic surveillance is mandatory and crucial to prevent the long-term effects of the COVID-19 pandemic, particularly bacterial infection.
Keywords: antibiotic resistance, bacterial infections, COVID-19, laboratory-based surveillance
Introduction
Coronavirus disease 2019 (COVID-19) is a new respiratory disease declared a pandemic in March 2020, and it is caused by a strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Furthermore, the pandemic has spread to many countries.1 Starting from March 2020 until beginning of January 2022, there were 317.389.048 positive cases worldwide and 4.262.994 in Indonesia. Meanwhile, 709.204 cases were reported from West Java.2 Dr. Hasan Sadikin General Hospital (RSHS) is a central hospital in this province with a maximum capacity of 1000 bed-inpatient. During the pandemic, RSHS changed its status to a COVID-19 referral hospital, which received an average of 291 patients per day.
The clinical presentation of COVID-19 varies from mild to severe and is influenced by patient comorbidities, including diabetes mellitus and hypertension.3 Previous studies identified problems with hospitalization and antibiotic usage in managing this virus.4,5 The hospitalization rate due to this pandemic was as high as 10%, with an average length of stay (LOS) of 9 days, among those without bacterial infection. However, the high number of hospitalized patients and LOS contribute to an increased risk of hospital-acquired infections.6 The other problem relates to antibiotic abuse during the COVID-19 pandemic, which affects bacterial resistance. Antibiotics are still prescribed in hospital settings for various reasons, despite that they are ineffective against the virus. The primary reasons for using antibiotics as an empirical treatment are the inability to differentiate between COVID-19 and bacterial infections in terms of severity.7,8
Before the pandemic outbreak, the World Health Organization (WHO) identified and designated antibiotic surveillance as one of the primary pillars in the fight against its resistance. The surveillance data are essential for evaluating the local antibiotic situation and providing evidence for developing the empirical guideline.9 However, there is an information gap about the prevalence and incidence of bacterial infection and antibiotic resistance among COVID-19 patients. A surveillance study was conducted in RSHS from March 2020 to October 2021 to describe the antibiotic resistance in a patient with a culture-proven bacterial infection.
Materials and Methods
Study Design and Patient Population
This retrospective study reviews the list of medical records of patients diagnosed with COVID-19 according to the International Classification of Diseases 10th Revision (ICD-10) Code U07.1 (COVID-19, virus identified) between March 2020 and October 2021 at Dr. Hasan Sadikin General Hospital Bandung, Indonesia.
All patients with COVID-19 were extracted from the hospital information system (Sistem Informasi Rumah Sakit Hasan Sadikin, Bandung, Indonesia) to identify the COVID-19 patient that has been confirmed by laboratory testing (ICD-10 Code U07.1). Furthermore, the list of confirmed patients merged with data on culture and antibiotic susceptibility from the laboratory information system (HCLAB Micro, Sysmex, Asia Pacific). Available clinical information from the patient list was obtained, and the patient was selected according to the inclusion and exclusion criteria. The inclusion criteria were (1) adult patients that are 18 years or above, (2) hospitalized with any degree of COVID-19 severity,10 and (3) submitted any clinical specimen (urine, blood, sputum, and pus) for culture. Meanwhile, the exclusion criteria were (1) re-hospitalized patients within the same period and (2) commensal bacterial (e.g. Viridans group streptococci, Micrococcus sp., Lactobacillus sp., Bacillus sp., Corynebacterium sp.) and fungi that grow on the clinical specimen. Finally, a hand search was conducted on medical records for information not available in the patient list.
The data on age, gender, type of ward, length of stay, clinical outcome, type of specimen, bacteria name, and antibiotics susceptibility results were collected for this study. The characteristic data were categorized as age group by decade, type of ward stratified into intensive and non-intensive, and clinical outcome into survive and non-survive.
Antibiotic Surveillance
Bacterial identification and antibiotic susceptibility testing (AST) were performed using an automatic microbiology analyzer (Vitek2 Compact, Biomerieux, France). The protocol for bacterial identification follows the WHO and Clinical and Laboratory Standards Institute (CLSI) guidelines.11–13 According to the CLSI guidelines, the AST result was validated and interpreted.13 In this surveillance study, an intermediate result of AST was considered as resistant. WHONET 5.6, a software recommended by the World Health Organization for Surveillance of Antimicrobial Resistance, was used to obtain the cumulative report of organism and AST.14,15 Furthermore, the surveillance rules were followed according to the Global Antimicrobial Resistance and Use surveillance system (GLASS) and CLSI recommendation.16,17 The antibiotics reported in this study were selected based on the American Thoracic Society Guideline for Pneumonia18 and CLSI guidelines13 for Gram-negative bacteria (GNB) and Gram-positive bacteria (GPB). Those reported for GNB were cefotaxime, ceftriaxone, ciprofloxacin, and ampicillin/sulbactam. Meanwhile, the antibiotics reported for GPB were oxacillin, linezolid, vancomycin, and levofloxacin. Finally, the selected antibiotic resistance percentage is considered high when equal to or more than 20%.19
Statistical Analysis
This study determined the prevalence of resistance to selected antibiotics among COVID-19 patients as a percentage of the number of bacteria tested, stratified by Gram-bacteria type. The patient characteristic data and the cumulative antibiotic result were summarized as frequencies and percentages using Microsoft Excel 2013 (Microsoft Corp.) and WHONET 5.6 (WHO Collaborating Centre for Surveillance of Antimicrobial Resistance). Furthermore, the bacteria distribution graph was drawn using STATA 12.0. (Stata, Texas, USA).
Results
Patient Characteristics
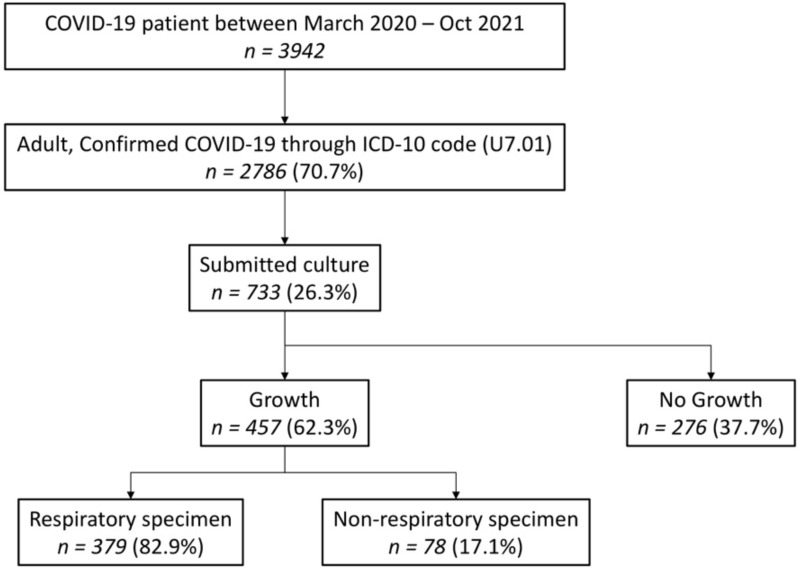
There were 2786 adult patients confirmed to have COVID-19 according to the ICD-10 U7.01, and 26.3% (n = 733) of them submitted clinical specimens for culture, out of which 62.3% (n = 457) showed positive growth, with 82.9% (n = 379) being dominated by the respiratory specimen. Figure 1 indicates that the prevalence of culture-proven bacterial infection in COVID-19 was 16.4% (457/2786). From the patient characteristic, 25% (n = 183) of adult admitted to the hospital was between 51 and 60 years old, with non-intensive ward type (72%, n = 529). Out of the study participants, 73.8% (n = 541) were discharged from the hospital with improved/survive clinical outcomes and a median hospital stay of 13 days (Table 1).
Figure 1.
Study flow chart.
Abbreviations: COVID-19, coronavirus disease 2019; ICD-10, The International Classification of Diseases 10th Revision; n, number of patients.
Table 1.
Patient Characteristics
| Variable | Total (n = 733) | |
|---|---|---|
| n | (%) | |
| Age group (years) | ||
| 18–20 | 7 | 1.0 |
| 21–30 | 63 | 8.6 |
| 31–40 | 109 | 14.9 |
| 41–50 | 138 | 18.8 |
| 51–60 | 183 | 25.0 |
| 61–70 | 159 | 21.7 |
| >70 | 74 | 10.1 |
| Gender | ||
| Female | 322 | 43.9 |
| Male | 411 | 56.1 |
| Ward type | ||
| Intensive | 204 | 27.8 |
| Non-intensive | 529 | 72.2 |
| Length of stay (days) | ||
| Median (Min-Max) | 13 (0–53) | |
| Clinical Outcome | ||
| Survive | 541 | 73.8 |
| Not survive | 192 | 26.2 |
Bacterial Characteristics
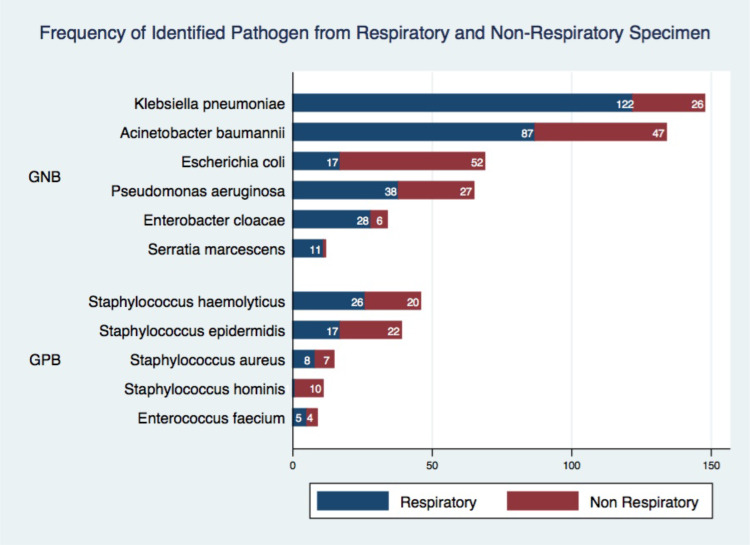
Gram-negative bacteria (GNB) were dominantly discovered among the respiratory and non-respiratory specimens. Klebsiella pneumoniae (122/148), Acinetobacter baumannii (87/134), and Pseudomonas aeruginosa (38/65) were commonly identified in the respiratory specimen. Meanwhile, Staphylococcus hominis (10/11) and Staphylococcus epidermidis (22/39), which were grouped into Coagulase-Negative Staphylococci (CoNS), are the most dominant Gram-positive bacteria (GPB) isolate among non-respiratory specimens (Figure 2).
Figure 2.
Distribution of identified pathogens from respiratory and non-respiratory specimen. X-axis shows the actual number of isolates identified and Y-axis shows the name of identified isolate, stratified by Gram-type bacteria.
Abbreviations: GNB, Gram-negative bacteria; GPB, Gram-positive bacteria.
Antibiotic Resistance Among Identified Isolates
High range resistance of beta-lactam combination (ampicillin-sulbactam, 24–100%), cephalosporins (ceftriaxone, 22–81%; cefotaxime, 22–73%) and fluoroquinolone (ciprofloxacin, 20–86%) among all the GNB are commonly used empirically as primary treatment in respiratory and non-respiratory infections. The resistance percentage of carbapenem (meropenem) among all GNB ranged between 2% and 13%, except for A. baumannii, which has high resistance against intravenous antibiotics, including carbapenem (meropenem, 85%). Table 2 shows that the prevalence of Extended-spectrum Beta-lactamases (ESBL) for K. pneumoniae and E. coli were 32% and 63%, respectively. The high resistance to levofloxacin ranged between 23% and 85%, and it is empirically used as the primary treatment for respiratory infections. In the CoNS group, resistance to oxacillin as the surrogate marker for identifying Methicillin-Resistance Staphylococci ranged between 77% and 92% in respiratory and non-respiratory specimens. Meanwhile, the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) was low, reaching 7%, in all specimens (Table 3).
Table 2.
Percentage Resistance of Gram-Negative Bacteria to Selected Antibiotics from Respiratory and Non-Respiratory Specimen
| Antibiotic Class | Antibiotic Agent | kpn | aba | eco | pae | ecl | sma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %R | n | %R | n | %R | n | %R | n | %R | n | %R | ||
| β-lactam combination | Ampicillin-Sulbactam | 148 | 24 | 134 | 82 | 69 | 60 | 65 | 100 | 34 | 100 | 12 | 100 |
| β-lactam combination | Piperacillin-tazobactam | 148 | 9 | 134 | 88 | 69 | 5 | 65 | 15 | 34 | 0 | 12 | 0 |
| Cephalosporin | Cefotaxime | 148 | 25 | 134 | – | 69 | 73 | 65 | – | 34 | 22 | 12 | 25 |
| Cephalosporin | Ceftriaxone | 148 | 25 | 134 | 81 | 69 | 75 | 65 | – | 34 | 35 | 12 | 22 |
| Cephalosporin | Ceftazidime | 148 | 21 | 134 | 84 | 69 | 29 | 65 | 17 | 34 | 18 | 12 | 22 |
| Cephalosporin | Cefepime | 148 | 11 | 134 | 87 | 69 | 22 | 65 | 15 | 34 | 5 | 12 | 0 |
| Aminoglycoside | Amikacin | 148 | 6 | 134 | 44 | 69 | 0 | 65 | 15 | 34 | 0 | 12 | 0 |
| Aminoglycoside | Gentamicin | 148 | 19 | 134 | 82 | 69 | 37 | 65 | 18 | 34 | 18 | 12 | 22 |
| Fluoroquinolone | Ciprofloxacin | 148 | 29 | 134 | 86 | 69 | 77 | 65 | 20 | 34 | 25 | 12 | 22 |
| Monobactam | Aztreonam | 148 | 32 | 134 | – | 69 | 63 | 65 | 21 | 34 | 22 | 12 | 22 |
| Folate pathway antagonist | Trimethoprim-sulfamethoxazole | 148 | 27 | 134 | 57 | 69 | 58 | 65 | – | 34 | 31 | 12 | 0 |
| Carbapenem | Meropenem | 148 | 10 | 134 | 85 | 69 | 2 | 65 | 13 | 34 | 6 | 12 | 0 |
Abbreviations: aba, Acinetobacter baumannii; ecl, Enterobacter cloacae; eco, Escherichia coli; kpn, Klebsiella pneumoniae; pae, Pseudomonas aeruginosa; sma, Serratia marcescens; n, number of isolates tested to a certain antibiotic agent; %R, percentage of antibiotic resistance; (-) dash, not tested.
Table 3.
Percentage Resistance of Gram-Positive Bacteria to Selected Antibiotics from Respiratory and Non-Respiratory Specimen
| Antibiotic Class | Antibiotic Agent | shl | sep | sau | sho | efm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %R | n | %R | n | %R | n | %R | n | %R | ||
| Aminoglycoside | Gentamicin | 46 | 64 | 39 | 25 | 15 | 0 | 11 | 10 | 9 | – |
| Lincosamide | Clindamycin | 46 | 80 | 39 | 58 | 15 | 7 | 11 | 90 | 9 | – |
| Macrolide | Erythromycin | 46 | 89 | 39 | 75 | 15 | 15 | 11 | 80 | 9 | 100 |
| Fluoroquinolone | Levofloxacin | 46 | 84 | 39 | 75 | 15 | 23 | 11 | 80 | 9 | 85 |
| Fluoroquinolone | Moxifloxacin | 46 | 71 | 39 | 58 | 15 | 16 | 11 | 70 | 9 | – |
| Oxazolidinone | Linezolid | 46 | 2 | 39 | 0 | 15 | 0 | 11 | 10 | 9 | 0 |
| Penicillin | Oxacillin | 46 | 92 | 39 | 77 | 15 | 7 | 11 | 90 | 9 | – |
| Glycopeptide | Vancomycin | 46 | 0 | 39 | 0 | 15 | 0 | 11 | 0 | 9 | 0 |
| Folate pathway antagonist | Trimethoprim-sulfamethoxazole | 46 | 46 | 39 | 72 | 15 | 7 | 11 | 80 | 9 | – |
Abbreviations: efm, Enterococcus faecium; sau, Staphylococcus aureus; sep, Staphylococcus epidermidis, shl, Staphylococcus haemolyticus; sho, Staphylococcus hominis; n, number of isolate tested to certain antibiotic agent; %R, percentage of antibiotic resistance; (-) dash, not tested.
Discussion
In terms of antibiotic resistance, bacterial infection in COVID-19 has become a source of concern and awareness. The empirical use of antibiotics in the early phase of the pandemic has affected and contributed to the increase in resistance.1 The bacterial infection in this virus was differentiated into co-infection and secondary bacterial infection, but this was challenging since a similar clinical manifestation was found between bacterial and viral pneumonia.20 This study reported that the overall prevalence of bacterial infection in COVID-19 was 16.4%. Meanwhile, it was reported previously to be 14.3%.4 During the observation, it was assumed that secondary bacterial infections likely occurred due to prolonged hospital stay and complications from SARS-CoV-2 infections. First, as previously reported, the median length of stay in this study was 13 days, which increased the risk of hospital-acquired infection.21 Second, in COVID-19, the SARS-CoV-2 virus damages the epithelial cells in the lower respiratory tract, which facilitates pathogenic bacteria to bind to the epithelial cell in conjunction with mucociliary dysfunction. Bacterial infection inhibits the repair and regeneration of the epithelial cell. This corresponds to the age group that suffers from the bacterial infection in this study, which was shown to be between 51 and 70 years. The decline in the function of the immune and respiratory system in adults, together with dysbiosis of respiratory microbiota, is a risk that facilitates the occurrence of bacterial infection in COVID-19.22,23
The Gram-negative bacteria (GNB), including K. pneumoniae, A. baumannii, P. aeruginosa, and E. coli, were dominantly identified in the respiratory and non-respiratory specimens from the COVID-19 patient. The previous study in the UK, Korea, and China showed similar results, indicating that GNB was the superior bacterial infection etiology among hospitalized patients.3,24,25 Before the pandemic, K. pneumoniae, A. baumannii, P. aeruginosa, and E. coli were listed as critical pathogens for antimicrobial resistance awareness, particularly carbapenem-resistant and third-generation cephalosporin-resistant.22 The study result shows high resistance to third-generation cephalosporin (cefotaxime, ceftriaxone) in K. pneumoniae, A. baumannii, E. coli, and carbapenem in A. baumannii. As previously stated, it is believed that the length of hospitalization, resulting in additional bacterial illnesses, is to blame for this similarity to the situation before the pandemic.20 Carbapenem-resistant A. baumannii (CR-Ab) should also be of concern. The previous study has reported poor clinical outcomes in patient management, especially in intensive care units.1,21,26 Additionally, the presence of these bacteria is related to a history of prolonged antibiotic use or treatment failure.21,27 The practice of frequent and prolonged use of antibiotics, particularly fluoroquinolone and third-generation cephalosporin, in Indonesian hospital settings has been identified and reported in the previous studies.28 Therefore, our study notes the limited choice of antibiotics that can be used empirically as an early treatment for bacterial infections in COVID-19. The resistance condition also applies to Gram-positive bacteria (GPB), of which high resistance among selected antibiotics was tested to Coagulase Negative Staphylococci (CoNS). The previous study indicates that CoNS should be considered because it can mediate resistance to S. aureus, particularly in immune-compromised, hospitalized, and elderly patients.29 However, the presence of Methicillin-resistant Staphylococcus aureus was low (below 10%) among the study population. However, this finding of high resistance isolate (GNB and GPB) in COVID-19 also indicates poor environmental control, such as poor hand hygiene, and poor infection control practices. During the COVID-19, the use of personal protective equipment for health workers has caused cross-contamination among hospitalized patient and affect to the nosocomial infection.27
There are several limitations in this study. First, we experience selection bias for bacterial culture in COVID-19. As previously mentioned, the selected specimen submitted only for severe cases was a potential bias for laboratory surveillance.30–32 Therefore, since the laboratory-based surveillance approach is being used, this potential issue seems unavoidable. Second, there is a limited number of certain isolates (eg, Serratia marcescens, Enterococcus faecium). However, due to the emergence of the bacterial infection in COVID-19 and the limited information available, particularly in Indonesia, it is still permissible to report a small number of isolates with careful interpretation. Third, due to the rapid progression of the virus and limited information among the included patient, the bacterial infection could not be stratified according to disease severity, type of infection (eg, hospital-acquired infection and bacterial superinfection) or patient management (eg, using mechanical ventilation or not).
Bacterial infection in COVID-19 has become a present and future awareness priority. The abuse of antibiotics, the growth in hospitalized patients with extended stays, and the increased risk of hospital-acquired illnesses have proven to be significant contributors to the rise in the resistance to bacterial infections. Continuing the deployment of antimicrobial surveillance is obligatory and essential; hence, it can assist in managing the trend of hospital antibiotic usage and establishing guidelines based on locally accessible data.
Conclusion
The prevalence of bacterial infection among COVID-19 patients was 16.4%, with Gram-negative bacteria predominating. The detected isolates were highly resistant to fluoroquinolones and cephalosporins, routinely used as first-line empirical treatments for respiratory and non-respiratory infections in Indonesia. Furthermore, the continuous monitoring of antimicrobial resistance with proper surveillance methods is mandatory and essential to prevent the long-term effect of the COVID-19 pandemic, particularly bacterial infection.
Ethical Approach
The study was conducted in accordance with the Declaration of Helsinki. This research protocol was approved by the Ethical Committee of Universitas Padjadjaran (1057/UN6.KEP/EC/2021) and permission to collect data was granted from the Ethical Committee of Dr. Hasan Sadikin Hospital (LB.02.01/X.2.2.1/5930/2022). Since the data were derived from the hospital and laboratory information system, no informed consent was required; therefore, the need for patient consent was waived by the ethical committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103–108. doi: 10.1016/j.ijid.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pusat Informasi & Koordinasi Provinsi Jawa Barat. Sebaran kasus Covid-19 di jawa barat. Pusat informasi & koordinasi provinsi jawa barat; 2022. Available from: https://pikobar.jabarprov.go.id/distribution-case. Accessed January 13, 2022.
- 3.Lv Z, Cheng S, Le J, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22(4–5):195–199. doi: 10.1016/j.micinf.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Bornman C, Zafer MM. Antimicrobial resistance threats in the emerging COVID-19 pandemic: where do we stand? J Infect Public Health. 2021;14(5):555–560. doi: 10.1016/j.jiph.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42(1):84–88. doi: 10.1017/ice.2020.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owoicho O, Tapela K, Djomkam Zune AL, Nghochuzie NN, Isawumi A, Mosi L. Suboptimal antimicrobial stewardship in the COVID-19 era: is humanity staring at a postantibiotic future? Future Microbiol. 2021;16(12):919–925. doi: 10.2217/fmb-2021-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 10.Kementerian Kesehatan RI. Pedoman pencegahan dan pengendalian coronavirus disease (COVID-19); 2020.
- 11.Vandepitte J; World Health Organization. Basic Laboratory Procedures in Clinical Bacteriology. 2nd ed. World Health Organization; 2003. [Google Scholar]
- 12.World Health Organization. Diagnostic Stewardship: A Guide to Implementation in Antimicrobial Resistance Surveillance Sites. World Health Organization; 2016. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Supplement M100. 30th ed. Clinical and Laboratory Standards Institute; 2020. [Google Scholar]
- 14.Vong S, Anciaux A, Hulth A, et al. Using information technology to improve surveillance of antimicrobial resistance in South East Asia. BMJ. 2017:j3781. doi: 10.1136/bmj.j3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AS, Karunaratne K, Shakya G, et al. Strengthening laboratory surveillance of antimicrobial resistance in South East Asia. BMJ. 2017:j3474. doi: 10.1136/bmj.j3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. World Health Organization; 2021. [Google Scholar]
- 17.Clinical Laboratory Standard Institute. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline. 3rd ed. Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 18.Metlay JP, Waterer GW, Long AC, et al.; Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indonesian Ministry of Health. Peraturan Menteri Kesehatan Republik Indonesia Nomor 8 Tahun 2015 Tentang Program Pengendalian Resistensi Antimikroba di Rumah Sakit; 2015.
- 20.Zhu X, Ge Y, Wu T, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect. 2021;27(12):1772–1776. doi: 10.1016/j.cmi.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Terán A, Mejía-Nepomuceno F, Herrera MT, et al. Dysbiosis and structural disruption of the respiratory microbiota in COVID-19 patients with severe and fatal outcomes. Sci Rep. 2021;11(1):21297. doi: 10.1038/s41598-021-00851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta. 2020;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Koh JS, Kim YJ, et al. Secondary infection among hospitalized COVID‐19 patients: a retrospective cohort study in a tertiary care setting. Respirology. 2021;26(3):277–278. doi: 10.1111/resp.13992 [DOI] [PubMed] [Google Scholar]
- 26.Townsend L, Hughes G, Kerr C, et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist. 2020;2(3):dlaa071. doi: 10.1093/jacamr/dlaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoso P, Sung M, Hartantri Y, et al. MDR pathogens organisms as risk factor of mortality in secondary pulmonary bacterial infections among COVID-19 patients: observational studies in two referral hospitals in West Java, Indonesia. Int J Gen Med. 2022;15:4741–4751. doi: 10.2147/IJGM.S359959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limato R, Nelwan EJ, Mudia M, et al. A multicentre point prevalence survey of patterns and quality of antibiotic prescribing in Indonesian hospitals. JAC Antimicrob Resist. 2021;3(2):dlab047. doi: 10.1093/jacamr/dlab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otto M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and. BioEssays. 2013;35(1):4–11. doi: 10.1002/bies.201200112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin TL, McNulty C, Beck C, MacGowan A. Antimicrobial resistance surveillance in urinary tract infections in primary care. J Antimicrob Chemother. 2016;71(10):2723–2728. doi: 10.1093/jac/dkw223 [DOI] [PubMed] [Google Scholar]
- 31.Laupland KB, Ross T, Pitout JDD, Church DL, Gregson DB. Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med. 2007;30(4):E159–E166. doi: 10.25011/cim.v30i4.1777 [DOI] [PubMed] [Google Scholar]
- 32.Rempel OR, Laupland KB. Surveillance for antimicrobial resistant organisms: potential sources and magnitude of bias. Epidemiol Infect. 2009;137(12):1665–1673. doi: 10.1017/S0950268809990100 [DOI] [PubMed] [Google Scholar]