Figure 1.
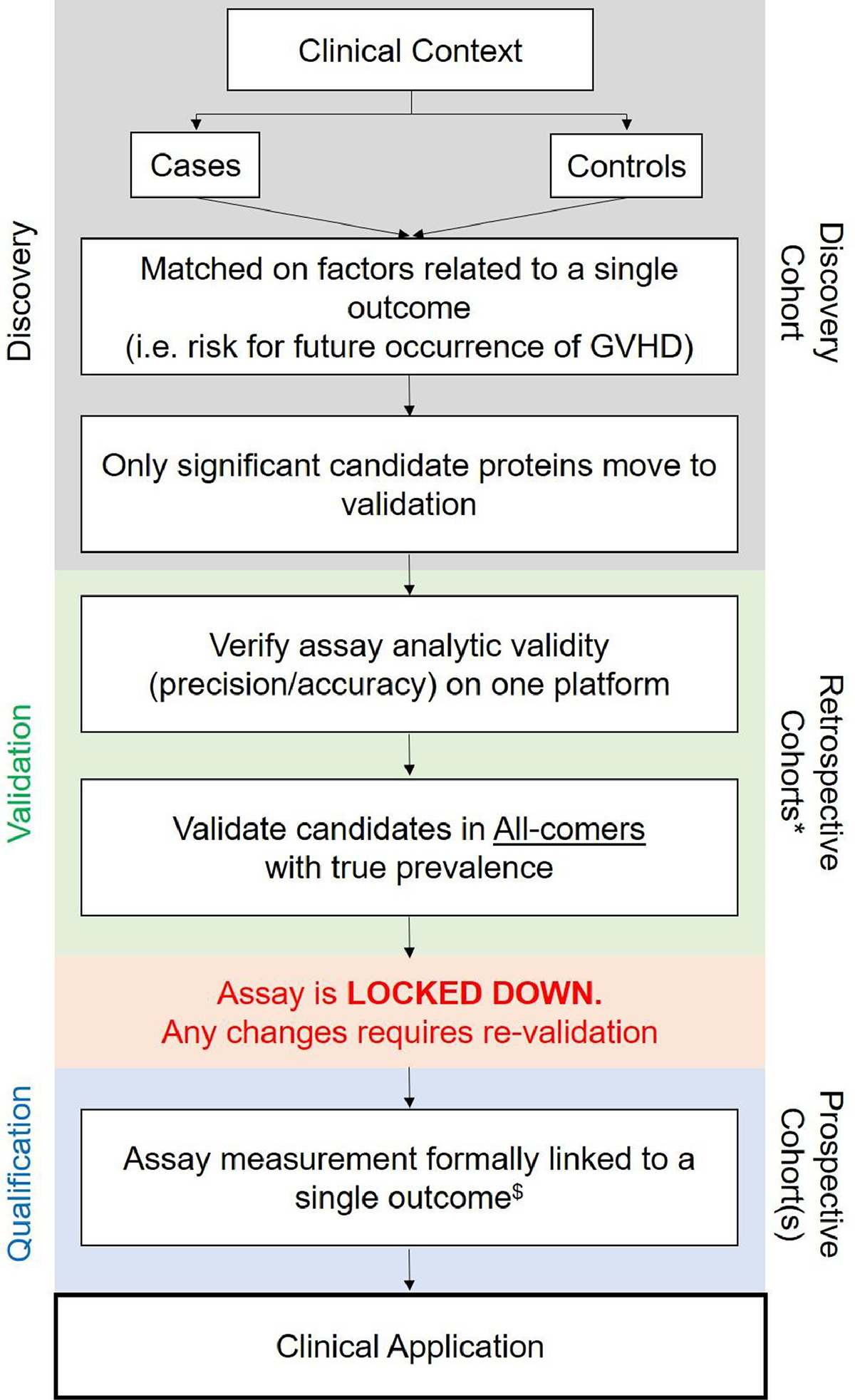
Recommended practices for biomarker development. *Validation requires evaluation in at least 2 independent cohorts.$Once a candidate protein reaches the qualification phase,it is called a biomarker of a specific type (see definitions).

Recommended practices for biomarker development. *Validation requires evaluation in at least 2 independent cohorts.$Once a candidate protein reaches the qualification phase,it is called a biomarker of a specific type (see definitions).