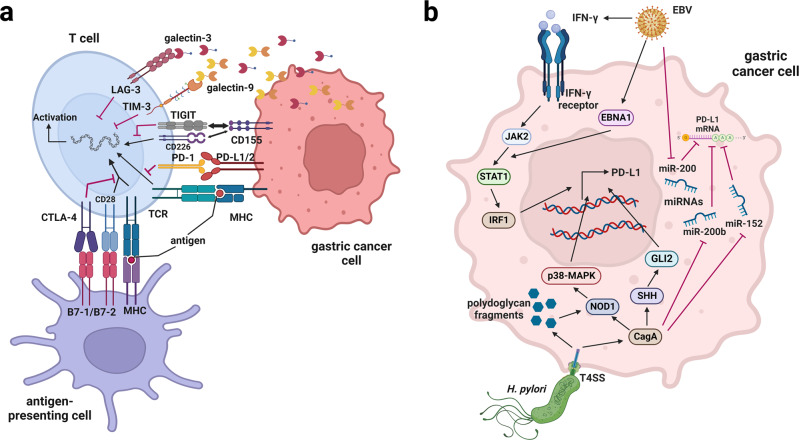
Fig. 3.
The immune checkpoint signaling pathways in gastric cancer and regulations on PD-L1 by H. pylori and EBV. a The immune checkpoint proteins PD-1 on the surface of T cells interact with the ligands PD-L1/PD-L2 on GC cells, or the aberrant CTLA-4 proteins on GC patient T cells interact with B7 on antigen-presenting cells, resulting in an immunosuppressive microenvironment, providing cancer cells with a survival advantage. TIGIT on the T cells membrane competes with the activation of CD226 binding to CD155 from the GC cells. Other immune checkpoint proteins, TIM-3 or LAG-3, interact with galectin-9 or galectin-3 released from GC cells, inhibiting the activation of T cells. b Chronic H. pylori or EBV infection, which are risk factors of GC, can induce upregulation of PD-L1 in GC cells via various signaling pathways and microRNAs, promoting immune escape. EBV Epstein–Barr virus, PD-1 programmed death 1, PD-L1/2 programmed death ligand 1/2, CTLA-4 cytotoxic T-lymphocyte-associated protein 4, TCR T-cell receptor, MHC major histocompatibility complex, TIGIT T cell immunoreceptor with Ig and ITIM domains, TIM-3 T cell immunoglobulin and mucin-domain containing-3, LAG-3 lymphocyte-activation gene 3, IFN-γ interferon gamma, JAK2 Janus kinase 2, STAT1 signal transducer and activator of transcription 1, IRF1 interferon regulatory factor 1, EBNA1 Epstein–Barr nuclear antigen 1, MAPK mitogen-activated protein kinase, NOD1 nucleotide-binding oligomerization domain-containing protein 1, SHH Sonic hedgehog protein, CagA cytotoxin-associated gene A, T4SS type IV secretion system. This figure was created with Biorender.com