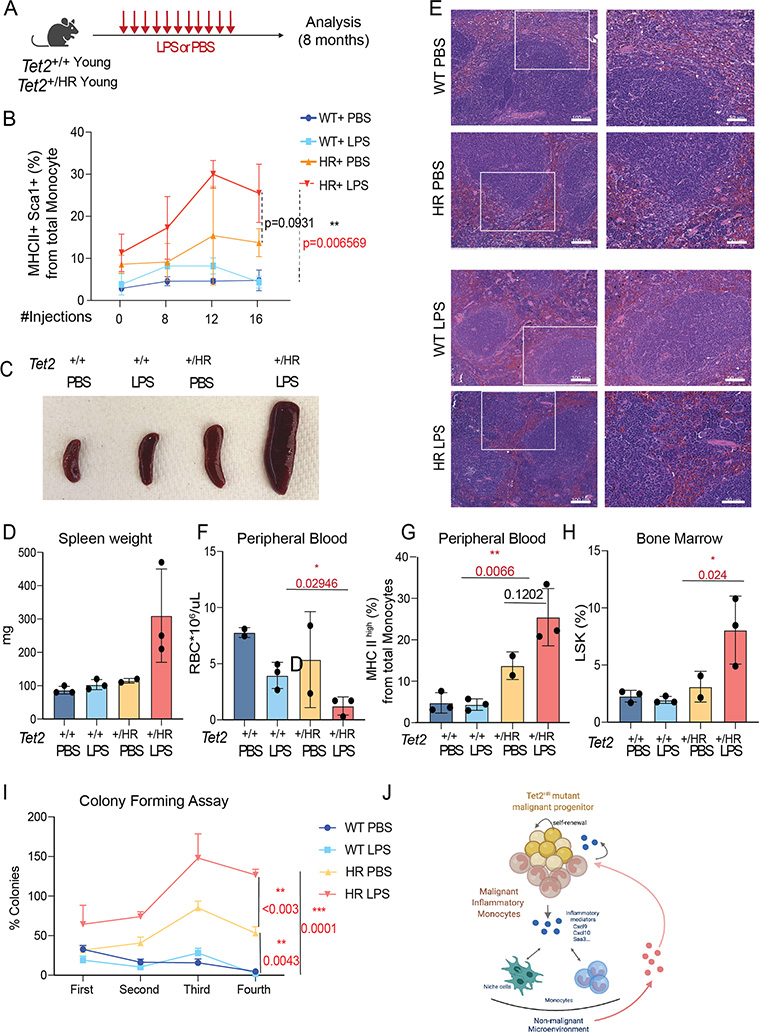
Figure 7. Inflammatory stimulation accelerates emergence of MHC IIhigh monocytes and leukemic transformation.
A, Experimental design of chronic LPS stimulation experiment. Tet2WT and Tet2HR young mice (8-weeks-old) were injected with 16 doses of 35 ug of LPS (every three days).
B, Percentage of MHC IIhigh Monocytes in the peripheral blood of the indicated mice during the LPS treatment described in A.
C, Representative spleens of Tet2WT and Tet2HR treated with PBS or LPS with LPS treated Tet2HR showing overt splenomegaly.
Experimental design. Bone marrow cells from Tet2HR mice with transformed disease were transplanted into recipients sub-lethally irradiated CD45.1.
D, Spleen weights of of Tet2WT and Tet2HR treated with PBS and LPS
E, Hematoxylin & Eosin of spleen sections of the indicated mice. No to minimal involvement observed in Tet2WT PBS and LPS treated samples. Chronic phase disease and signs of extramedullary MPN/MDS-MPN observed in Tet2HR LPS treated samples.
F, Hemavet results showing red blood count (RBC) in Tet2WT and Tet2HR PBS and LPS treated mice
G, Percentage of MHC IIhigh Monocytes in the peripheral blood of the indicated mice 8 months following LPS treatment.
H, Percentage of LSK in bone marrow of old Tet2 +/+ or old Tet2+/HR with transformed disease phenotype quantified by flow cytometry immunophenotyping.
I, Colony formation assay. Quantification of the number of colonies derived from serial plating of 10,000 unfractionated bone marrow cells collected from PBS and LPS treated Tet2 WT and Tet2 +/HR (n=3).
J, Tet2 mutant progenitors maintain a tight regulation through their hematopoietic differentiation during steady-state. However, inflammatory conditions such as aging and infections triggers the proliferation of the progenitor cells and their myeloid-bias differentiation into inflammatory monocytes. The uncontrolled inflammatory response, in part maintained by the Tet2HR inflammatory monocyte contributes to the transformation of the progenitor cells and the sustained generation of leukemic inflammatory monocyte