Figure 1.
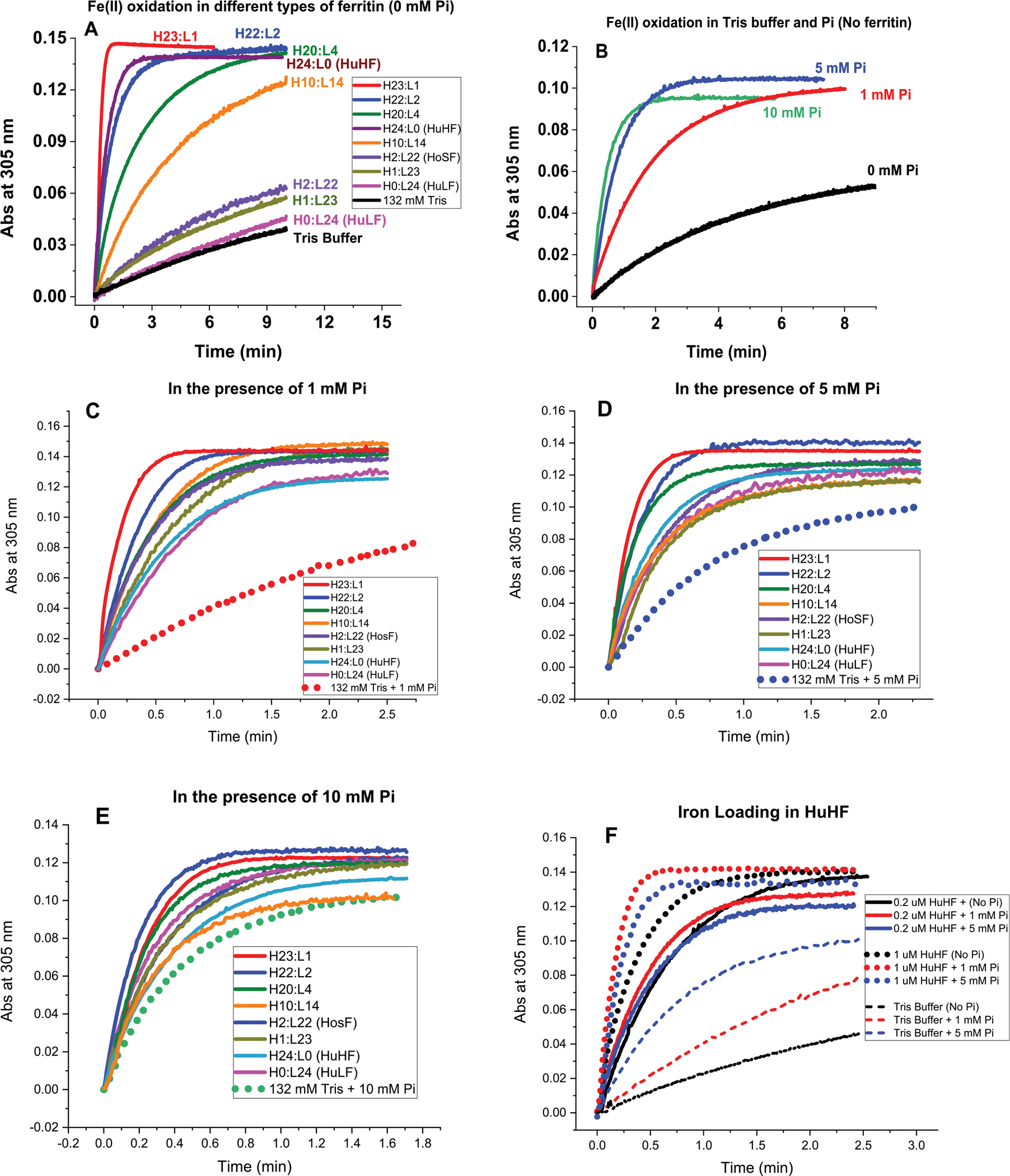
Iron oxidation kinetics at 305 nm in the presence and absence of phosphate using light absorption spectroscopy. Conditions (A–E): 132 mM Tris buffer with 0, 1, 5, or 10 mM sodium phosphate; 0.2 μM ferritin; 40 μM FeSO4; pH 7.4; and 25.0 °C. Panel F displays the iron oxidation kinetics of recombinant human H-ferritin at 0.2 and 1 μM ferritin in 1 and 5 mM phosphate compared to Tris buffer. Panel F: effect of ferritin concentration on iron loading into human H-ferritin. We note that the number of H and L subunits in any heteropolymer ferritin is likely an average value representing a heterogeneous mixture of heteropolymers whose H and L subunit composition could vary between 5 and 8%.
