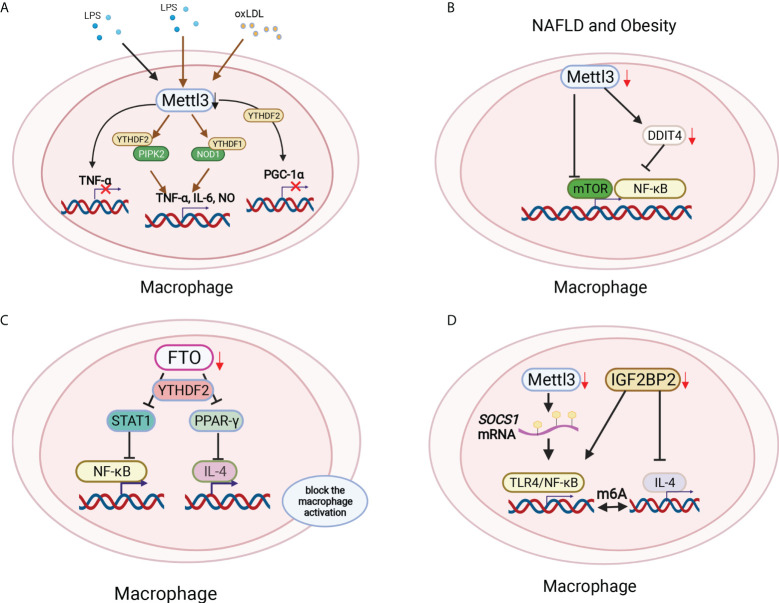
Figure 4.
Macrophage polarization was modified by m6A methylation in different environments. (A) Mettl3 ablation macrophages produced little TNF-α with LPS stimulation. Mettl3 downregulation in macrophages significantly increased the proinflammatory cytokines TNF-α, IL-6 and NO by increasing NOD1 and RIPK2 via YTHDF1 and YTHDF2, respectively. Mettl3 and YTHDF2 cooperatively degraded PGC-1α mRNA in oxLDL-treated monocytes. (B) Myeloid lineage-restricted Mettl3 deletion protected mice from age-related and diet-induced development of innate immunity-driven nonalcoholic fatty liver disease (NAFLD) and obesity by decreasing mTOR expression and the NF-κB pathway by targeting DDIT4. (C) FTO deficiency inhibited both M1 and M2 polarization by suppressing the NF-κB signaling pathway and decreasing the stability of STAT and PPAR-γ via YTHDF2 involvement, therefore blocking macrophage activation. (D) The loss of Mettl14 decreased the demethylase Socs1 mRNA to activate TLR4/NF-κB signaling. In addition, IGF2BP2-/- macrophages were refractory to IL-4-induced activation to regulate the switch of M1 to M2 subtypes in a m6A-dependent manner.