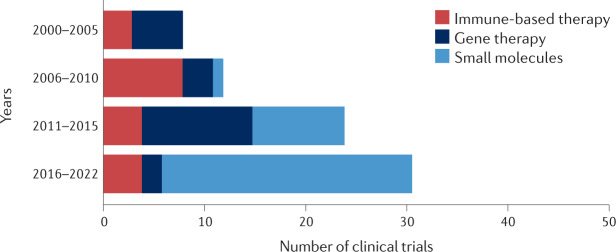
Fig. 2. Numbers of p53-targeted clinical trials by year and treatment category.
Clinical trials with p53-targeted therapies initiated after 1 January 2000 were stratified by year blocks and category. Gene therapy clinical trials were popular before the year 2000 (12 clinical trials initiated), but their number declined sharply soon thereafter, owing to mounting concerns about the safety of this strategy. These numbers increased again in the course of 2011–2015, mostly reflecting the clinical trials driven by Shenzhen SiBiono GeneTech and several trials of SGT-53 (SynerGene Therapeutics). Immune-based clinical trials targeting p53 were rather uncommon before 2000 (two clinical trials). With the introduction of new anticancer vaccination approaches, the number of relevant p53-based clinical trials has increased. Presently, most p53-based immunotherapy clinical trials use a combination of immune checkpoint inhibition and a p53-activating agent (either gene therapy or small molecules). It is expected that, owing to the growing interest in bispecific antibodies and T cell receptor (TCR)-like antibodies (see Fig. 4), p53-centric clinical trials that use these strategies will become more popular in the coming years. Visibly, the biggest increase in p53-based clinical trials in the past decade involved small-molecule drugs. This may be attributed, at least in part, to the emergence of new screening methods and improved compound libraries, along with better understanding of the deregulation of p53 in cancer.