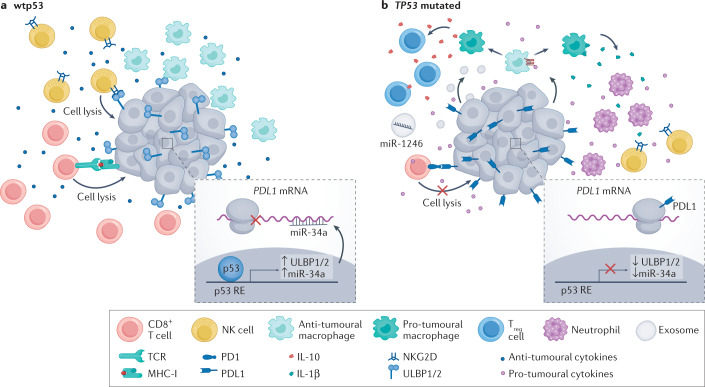
Fig. 5. p53 can influence immunotherapy by modulating the tumour immune microenvironment.
In p53-wild-type (wtp53) tumours, the tumour immune microenvironment has a predominantly anti-tumoural effect. This can be attributed to several mechanisms. For example, wtp53 can upregulate the expression of UL16-binding protein 1 (ULBP1) and ULBP2 and microRNA miR-34a. ULBP1 and ULBP2 are activating ligands for natural killer (NK) cells, which enhances their cytotoxic activity. miR-34 blocks the translation of PDL1 mRNA and drives its degradation; consequently, PDL1 protein levels are downregulated in cancer cells that retain wtp53, sensitizing these tumour cells to CD8+ T cell-mediated killing. Moreover, wtp53 can promote the secretion of anti-tumoural cytokines such as tumour necrosis factor (TNF), which further enhances the protective effects of the tumour immune microenvironment. In TP53-mutated cells, ULBP1 and ULBP2 are downregulated and PDL1 is upregulated, causing resistance to killing by NK and CD8+ T cells, respectively. Furthermore, loss of p53 function can drive secretion of WNT ligands, which bind to their cognate receptors on macrophages, causing them to secrete IL-1β, which attracts pro-tumoural cells such as neutrophils. Mutp53 proteins promote the secretion of exosomes that can deliver microRNAs such as miR-1246, which convert anti-tumoural M1 macrophages to pro-tumoural M2 macrophages. Such pro-tumoural macrophages secrete IL-1β, which attracts pro-tumoural regulatory T (Treg) cells that dampen the anti-tumoural response and protect the tumour from immune elimination. MHC-I, major histocompatibility complex class I; RE, response element; TCR, T cell receptor.