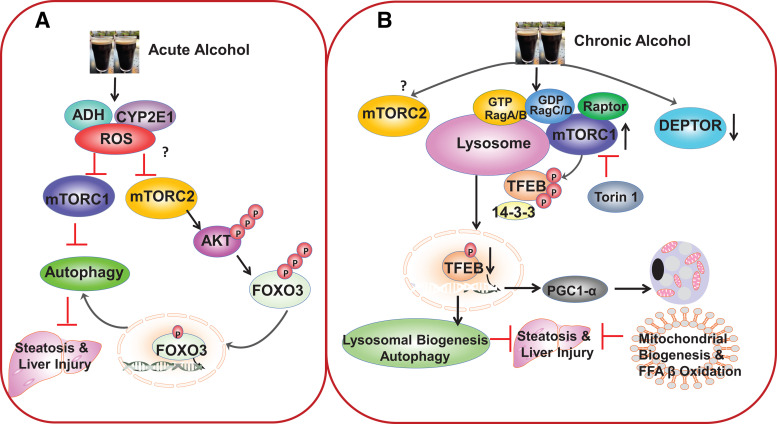
Figure 2.
Acute and chronic alcohol exposure have distinctive effects on mechanistic target of rapamycin complex 1 (mTORC1) and autophagy in mouse livers. A: acute ethanol binge induces autophagy by inhibiting mTORC1 and mechanistic target of rapamycin complex 2 (mTORC2). Ethanol is metabolized in the liver by alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1) to increase reactive oxygen species (ROS) production, which may lead to the inhibition of both mTORC1 and mTORC2 by yet unknown mechanisms. Decreased mTORC1 then promotes hepatic autophagy induction. Decreased mTORC2 leads to decreased protein kinase B (AKT) phosphorylation and activation resulting in decreased Forkhead box O-3 (FOXO3) phosphorylation and increased FOXO3 nuclear translocation. Activated FOXO3 increases the expression of autophagy related genes and autophagy induction to protect against acute alcohol-induced liver injury. B: chronic feeding plus acute ethanol binge impairs autophagy by increasing mTORC1 activation. Chronic feeding plus acute ethanol binge increases lysosomal RagA and decreases DEP-domain-containing mTOR-interacting protein (DEPTOR) resulting in mTOR lysosomal translocation and mTORC1 activation. Increased mTORC1 activation promotes the phosphorylation and cytosolic retention of transcription factor EB (TFEB) resulting in decreased nuclear TFEB translocation. Decreased nuclear TFEB reduces the expression of lysosomal and –autophagy-related genes as well as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) the master regulator of mitochondrial biogenesis. Torin1 inhibits mTORC1 and rescues alcohol-induced defective TFEB resulting in increased biogenesis of lysosomes and mitochondria as well as autophagy to protect against alcohol-induced liver injury. Whether chronic ethanol feeding would affect mTORC2 remains to be determined.