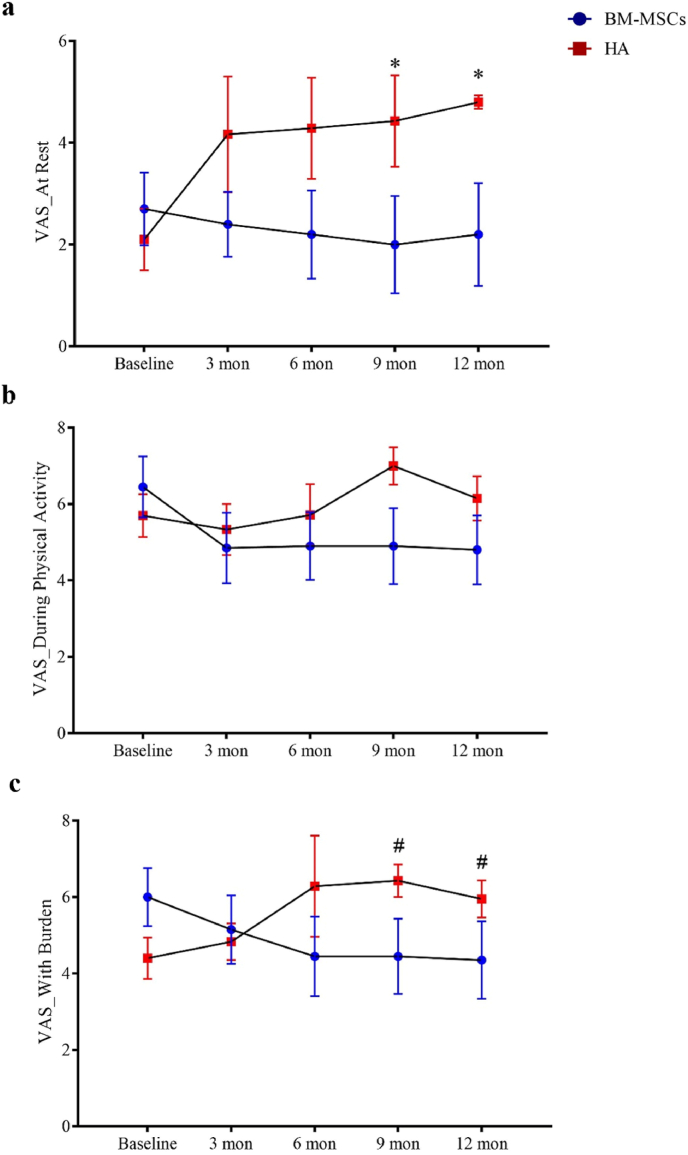
Figure 3.
Changes of VAS pain score during the one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square). VAS was determined a) at rest, b) during physical activity or c) with burden at baseline, 3 months, 6 months, 9 months, and 12 months. There was a trend of pain reduction in participants which received BM-MSCs injection when compared with those who received HA injection. Significant reduction in rest pain and in pain with burden at 9 months and 12 months post injection was found in BM-MSCs group. Data shown as mean ± standard deviation. ∗P < 0.05 in Mann-Whiney U test. #P < 0.05 in Bonferroni's correction multiple comparisons test. VAS: visual analogue scale; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid; Mon, month(s).