Abstract
Background
Acetyl‐coenzyme A carboxylase 1 (ACC1) regulates lipid homeostasis, T helper (Th) cell differentiation, oxidative stress, inflammation response, and neurological process, engaging in acute ischemic stroke (AIS) pathogenesis, while its clinical utility in AIS is unclear. Hence, this study intended to explore the correlation among blood ACC1, Th17, and Th1 cells, and ACC1’s potency as a prognostic biomarker for AIS management.
Methods
ACC1 in peripheral blood mononuclear cells (PBMCs) of 160 AIS patients and 30 controls were determined using RT‐qPCR; blood Th17 and Th1 cells in AIS patients were quantified by flow cytometry.
Results
ACC1 was increased in AIS patients compared with controls (median (interquartile range): 2.540 (1.753–3.548) vs. 0.980 (0.655–1.743), p < 0.001), which exhibited a good value to reflect AIS risk with the area under the curve of 0.872 (95% CI: 0.805–0.939). Moreover, ACC1 was positively linked with Th17 (r = 0.374, p < 0.001) and Th1 (r = 0.178, p = 0.024) cells in AIS patients. Additionally, ACC1 (r = 0.328, p < 0.001), Th17 (r = 0.272, p = 0.001), and Th1 cells (r = 0.195, p = 0.014) were positively associated with the National Institutes of Health Stroke Scale score in AIS patients. ACC1 high vs. low (p = 0.038) and Th17 high vs. low (p = 0.026) were related to shortened recurrence‐free survival (RFS) in AIS patients, while Th1 cells (p = 0.179) were not correlated with RFS. Whereas ACC1 (p = 0.248), Th17 (p = 0.079), and Th1 cells (p = 0.130) were not linked with overall survival (OS) in AIS patients.
Conclusion
Circulating ACC1 overexpression correlates with increased Th17, Th1 cells, NIHSS score, and shortened RFS in AIS patients.
Keywords: acetyl‐coenzyme A carboxylase 1, acute ischemic stroke, NIHSS score, survival, Th17 and Th1 cells
Blood acetyl‐coenzyme A carboxylase 1 (ACC1) of 160 acute ischemic stroke (AIS) patients and 30 controls was determined using RT‐qPCR; blood T helper (Th)1 and Th17 cells in AIS patients were quantified by flow cytometry. ACC1 was increased in AIS patients compared with controls (P<0.001). ACC1 was positively linked with Th1 (P=0.024) and Th17 (P<0.001) cells in AIS patients. ACC1 (P<0.001), Th1 (P=0.014), and Th17 (P=0.001) cells were positively associated with the National Institutes of Health Stroke Scale (NIHSS) score in AIS patients. ACC1 high (P=0.038) and Th17 high (P=0.026) were related to shortened recurrence‐free survival (RFS) in AIS patients. Collectively, circulating ACC1 overexpression correlates with increased Th1, Th17 cells, NIHSS score, and shortened RFS in AIS patients.
1. INTRODUCTION
Acute ischemic stroke (AIS), representing nearly 70% of all stroke cases, is a common life‐threatening cerebrovascular disease with 24.2 million prevalent cases in 2019, whose mortality rate has increased by 32.3% over the past 30 years in China. 1 , 2 , 3 The occurrence of AIS is mainly attributed to cerebral vascular occlusion and inadequate blood supply, which are induced by dyslipidemia, chronic inflammation, atherosclerotic plaques, etc. 4 , 5 , 6 Although great efforts have been paid to restore vascular and prevent secondary neuronal injury, the prognosis of AIS patients is not pleasant, with a 1‐year recurrent rate of about 5.4% and a disability rate ranging from 26% to 50%. 7 , 8 , 9 Therefore, identifying potential biomarkers to monitor disease severity and prognosis is helpful for AIS management.
Acetyl‐coenzyme A carboxylase 1 (ACC1) is a cytoplasmic protein that participates in several biological processes (including lipid metabolism, neuronal apoptosis, T cell differentiation, inflammatory response, and oxidative stress). 10 , 11 , 12 , 13 For instance, one study shows that ACC1 mediates fatty acid synthesis and elevates human plasma triglycerides, which further causes dyslipidemia. 10 Also, a previous study discloses that ACC1 regulates T‐cell balance via regulating de novo fatty acid biosynthesis. 14 Besides, another study indicates that ACC1 facilitates atherosclerotic injury in ApoE−/− mice. 11 Considering that the aforementioned dyslipidemia, Th17/Treg imbalance, and atherosclerotic injury are all crucial pathogenic factors of AIS, it is speculated that ACC1 might be an important regulator in the development of AIS. 15 , 16 Nevertheless, only one study suggests that ACC1 knockdown protects against stroke injury in mice, while its clinical role needs further exploration. 17
Hence, this study was conducted intending to explore the relationship of blood ACC1 with Th17, Th1 cells, and its potency serving as a biomarker for AIS management.
2. METHODS
2.1. Subjects
One hundred sixty newly diagnosed AIS patients treated from June 2017 to May 2020 were serially recruited in this study. The enrollment criteria were (a) diagnosis of AIS per the American Stroke Association Guideline 18 ; (b) over 18 years old; (c) absence of intracranial hemorrhage based on imaging results; (d) voluntary for peripheral blood (PB) sample collection and data documentation. The exclusion criteria were (a) concomitant with infections, inflammatory diseases, or autoimmune diseases; (b) with a prior history of cancers or hematological malignancies; (c) during pregnancy or breastfeeding. Additionally, this study also included 30 subjects at high risk of stroke as controls (The high‐stroke‐risk factors were defined in a previous study 19 ), who had the following conditions were eligible for inclusion: (a) had no history of stroke; (b) had at least two high‐risk factors of stroke (including overweight, smoke, drink, chronic kidney disease, hyperuricemia, hypertension, diabetes, hyperlipidemia, hyperhomocysteinemia, and cardiovascular disease); (c) over 18 years old; (d) had no infections, inflammatory diseases, autoimmune diseases, solid tumors, and hematological malignant diseases; (e) non‐pregnant and non‐lactating. The study had the Ethics Committee's approval. The informed consents were collected in written or tape‐recording forms from the subjects or their families.
2.2. Data collection
Clinical features of AIS patients were gained, which included demographics, history of underlying disease, time since symptom to admission, and National Institutes of Health Stroke Scale (NIHSS) score at admission.
2.3. Sample processing and examination
PB sample was obtained from each AIS patient and each control after enrollment, and PBMC was separated. The isolated PBMC samples were used to detect ACC1 expression by RT‐qPCR. In brief, PureZOL RNA isolation reagent (Bio‐Rad) was applied for total RNA extraction; then, reserve transcription was finished by PrimeScript™ RT reagent Kit (Takara). Subsequently, the qPCR reaction was completed by KOD SYBR® qPCR Mix (TOYOBO). The relative expression was evaluated using the 2−ΔΔCt method, and 18S ribosomal RNA (rRNA) was used as the internal reference. 20 The design of qPCR primer sequences of ACC1 and 18S rRNA forward were as follows: 5’–CGCTATGGAAGTCGGCTGTG–3′; reverse: 5’–CAGGAAGAGGCGGATGGGAA–3′; 5’–TGAGAAACGGCTACCACATC–3′; Reverse: 5’–TTACAGGGCCTCGAAAGAGT–3′. Besides, AIS patients' PB samples were also used for detecting the percentages of Th17 and Th1 cells in CD4+ T cells through flow cytometry (FCM) using the Human Th1/Th17 Phenotyping Kit (ThermoFisher) per the instruction.
2.4. Follow‐up
Standard follow‐up of AIS patients was performed by clinic visit with the deadline of November 30, 2021. Recurrence‐free survival (RFS) and overall survival (OS) were imputed. RFS was identified as the duration between admission and disease recurrence or patient's death; OS was identified as the duration between admission and patient's death.
2.5. Statistics
Statistics were performed using spss 24.0 (IBM Corp.). Graphs were made using graphpad prism 6.01 (GraphPad Software Inc.). Comparison of ACC1 expression between different subjects was analyzed using Wilcoxon rank‐sum test, and the ability of ACC1 in differentiating AIS from controls was determined using receiver operating characteristic (ROC) curve. Association analysis of two variables was completed using Spearman's rank correlation test. RFS and OS were described using Kaplan–Meier curves and evaluated by log‐rank test. For survival analysis, ACC1 expression, Th17 cells, and Th1 cells were respectively divided into low and high groups based on median values in AIS patients. Comparison of ACC1 expression with clinical features was completed using Wilcoxon rank‐sum test. Prognostic factor analysis was completed using step‐forward multivariate Cox's proportional hazard regression model. p < 0.05 was considered significant.
3. RESULTS
3.1. Clinical features of AIS patients
The entire 160 AIS patients with a mean age of 64.6 ± 9.2 years consisted of 42 (26.3%) females and 118 (73.7%) males (Table 1). With regard to the disease history, 84 (52.5%), 134 (83.7%), 36 (22.5%), 30 (18.8%), and 47 (29.4%) patients suffered from hyperlipidemia, hypertension, diabetes, chronic kidney disease, and cardiovascular disease, respectively. The median (interquartile range [IQR]) time since symptom to admission was 4.0 (3.0–6.0) h. Besides, the mean NIHSS score was 8.2 ± 4.9. Concerning the treatment information, 130 (81.3%) patients received thrombolysis, and the other 30 (18.7%) patients underwent mechanical thrombectomy. The specific clinical features of AIS patients are displayed in Table 1.
TABLE 1.
Clinical features of AIS patients
| Items | AIS patients (N = 160) |
|---|---|
| Age (years), mean ± SD | 64.6 ± 9.2 |
| Gender, No. (%) | |
| Female | 42 (26.3) |
| Male | 118 (73.7) |
| BMI (kg/m2), mean ± SD | 24.3 ± 2.6 |
| History of smoke, No. (%) | |
| No | 71 (44.4) |
| Yes | 89 (55.6) |
| History of hypertension, No. (%) | |
| No | 26 (16.3) |
| Yes | 134 (83.7) |
| History of hyperlipidemia, No. (%) | |
| No | 76 (47.5) |
| Yes | 84 (52.5) |
| History of diabetes, No. (%) | |
| No | 124 (77.5) |
| Yes | 36 (22.5) |
| History of chronic kidney disease, No. (%) | |
| No | 130 (81.2) |
| Yes | 30 (18.8) |
| History of cardiovascular disease, No. (%) | |
| No | 113 (70.6) |
| Yes | 47 (29.4) |
| Time since symptom to admission (hours), median (IQR) | 4.0 (3.0–6.0) |
| NIHSS score, mean ± SD | 8.2 ± 4.9 |
| Treatment, No. (%) | |
| Thrombolysis | 130 (81.3) |
| Mechanical thrombectomy | 30 (18.7) |
Abbreviations: AIS, acute ischemic stroke; BMI, body mass index; IQR, interquartile range; NIHSS, National Institute Health of Stroke Scale; SD, standard deviation.
3.2. ACC1 in AIS patients and controls
ACC1 was up‐regulated in AIS patients (median (IQR): 2.540 (1.753–3.548), ranging from 0.850 to 8.130) compared with controls (median (IQR): 0.980 (0.655–1.743), ranging from 0.310 to 2.740) (p < 0.001, Figure 1A). Meanwhile, ACC1 exhibited a delightful value to differentiate AIS patients from controls (area under the curve (AUC): 0.872, 95% confidence interval (CI): 0.805–0.939) with the best cutoff point value of 1.345 (sensitivity: 0.869, specificity: 0.700) (Figure 1B).
FIGURE 1.
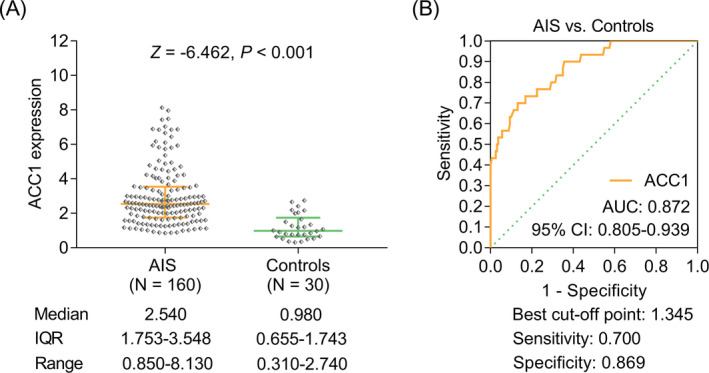
ACC1 was overexpressed in AIS patients than in controls. Comparison of ACC1 between AIS patients and controls (A). The value of ACC1 in distinguishing AIS patients and controls (B).
3.3. Linkage of ACC1 with clinical features in AIS patients
ACC1 expression was elevated in patients with a history of cardiovascular disease (vs. no) (p = 0.049), patients who were admitted >4 h since symptom (vs. ≤4 h) (p < 0.001), and patients treated with mechanical thrombectomy (vs. thrombolysis) (p = 0.011) (Table S1).
3.4. Linkage of ACC1 with Th17, Th1 cells, and their correlation with NIHSS in AIS patients
Among all 160 AIS patients, ACC1 was positively linked with Th17 (r = 0.374, p < 0.001, Figure 2A) and Th1 cells (r = 0.178, p = 0.024, Figure 2B). Additionally, ACC1 (r = 0.328, p < 0.001, Figure 3A), Th17 cells (r = 0.272, p = 0.001, Figure 3B), and Th1 cells (r = 0.195, p = 0.014, Figure 3C) were all positively related to the NIHSS score in AIS patients.
FIGURE 2.
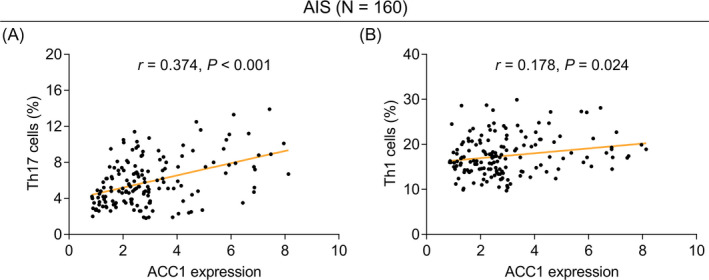
Elevated ACC1 was linked with increased Th17 and Th1 cells in AIS patients. Linkage of ACC1 with Th17 (A) and Th1 (B) cells in AIS patients.
FIGURE 3.
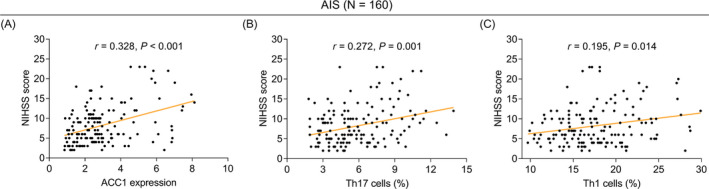
Elevated ACC1, Th17, and Th1 cells were linked with increased NIHSS score in AIS patients. Linkage of ACC1 (A), Th17 (B), and Th1 (C) cells with the NIHSS score in AIS patients.
3.5. Linkage of ACC1, Th17, and Th1 cells with RFS and OS in AIS patients
The last follow‐up date was November 30, 2021, with the median (95% CI) follow‐up of 25.0 (21.0–32.8) months (ranging: 3.0–50.0 months). During the follow‐up duration, 25 (15.6%) recurrences and 9 (5.6%) deaths occurred in AIS patients.
ACC1 high (vs. low) (p = 0.038, Figure 4A) and Th17 high (vs. low) (p = 0.026, Figure 4B) were related to shortened RFS in AIS patients, while Th1 cells (p = 0.179, Figure 4C) were not correlated with RFS. Whereas, ACC1 (p = 0.248, Figure 5A), Th17 cells (p = 0.079, Figure 5B), and Th1 cells (p = 0.130, Figure 5C) were not linked with OS in AIS patients.
FIGURE 4.
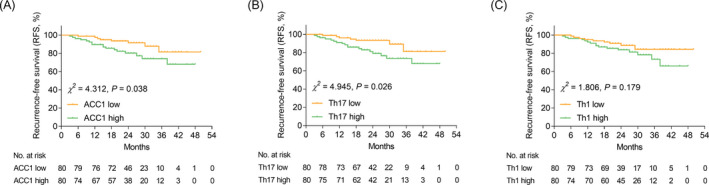
ACC1 high (vs. low) and Th17 cell high (vs. low) were linked with shortened RFS in AIS patients. Comparison of RFS between ACC1 high vs. low AIS patients (A). Comparison of RFS between Th17 high vs. low AIS patients (B). Comparison of RFS between Th1 high vs. low AIS patients (C).
FIGURE 5.
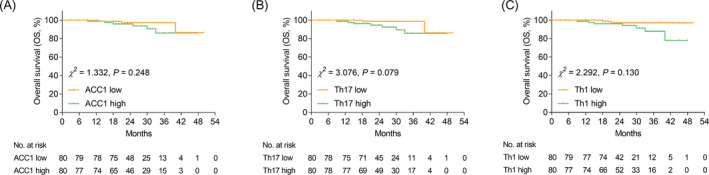
ACC1, Th17, and Th1 cells were not related to OS in AIS patients. Comparison of OS between ACC1 high vs. low AIS patients (A). Comparison of OS between Th17 high vs. low AIS patients (B). Comparison of OS between Th1 high vs. low AIS patients (C).
For further confirm the correlation of ACC1 expression with RFS and OS, the multivariate Cox's proportional hazards regression analysis was performed, which showed that the elevated ACC1 expression was independently linked with shortened RFS (hazard ratio [HR]: 1.242, 95% CI: 1.002–1.539, p = 0.048) and OS (HR: 1.423, 95% CI: 1.039–1.949, p = 0.028) in AIS patients (Table S2).
Additionally, time since symptom to admission >4 h (vs. ≤4 h) was related to declined RFS (p < 0.001, Figure S1A) and exhibited a correlating trend (without statistical significance) with decreased OS (p = 0.066, Figure S1B) in AIS patients.
4. DISCUSSION
ACC1, mainly acting on the fatty acid synthesis and fatty acid oxidation, is observed to involve in metabolic syndrome (including atherogenic dyslipidemia, diabetes, and hypertension), which is a clustering of cardiovascular/cerebrovascular risk factors. 21 , 22 , 23 , 24 For instance, one previous study shows that the overexpression of ACC1 facilitates lipid accumulation in macrophage foam cells and further aggravates atherosclerosis in apolipoprotein E‐deficient mice. 25 Also, another study finds that ACC1 expression is elevated in atherosclerotic mice. 11 However, the relevant study which determines ACC1 expression in AIS patients is still rare. The present study disclosed that ACC1 was overexpressed in AIS patients than that in controls; meanwhile, ACC1 exhibited a good value to reflect AIS risk. The probable explanation might be as follows: (1) ACC1 exacerbated lipid accumulation, which would further cause atherosclerotic plaque and increased the risk of cerebral vascular occlusion. 26 , 27 , 28 (2) ACC1 induced the inflammation level and oxidative stress, which would aggravate cerebral injury in AIS. 29 , 30 Combining the above aspects, ACC1 showed a good value to reflect AIS risk.
Regarding the linkage of ACC1 with T cells' differentiation, one previous study exhibits that ACC1 impacts the metabolic programming of T cells and subsequently stimulates CD4+ T cells differentiating into Th17 cells through the endogenous fatty acid synthesis pathway. 14 Besides, another study discloses that ACC1 causes Th17/Treg imbalance in AIS mice. 17 From the clinical aspect, the present study showed that ACC1 was positively linked with Th17 and Th1 cells in AIS patients. The probable explanation might be as follows: ACC1 activated T cells and promoted T cells differentiating into Th17 and Th1 cells. 14 As a result, increased ACC1 was related to elevated Th17 cells and Th1 cells in AIS patients. Additionally, it was also noticed that ACC1, Th17 cells, and Th1 cells were positively linked with the NIHSS score in AIS patients, which could be explained as follows: (1) ACC1 induced vascular endothelial cell impairment, whose aggravated injury was related to elevated disease severity in AIS patients. 26 , 31 Therefore, up‐regulated ACC1 was associated with increased disease severity in AIS patients. (2) ACC1 elevated plasma triglycerides, which would further enlarge the atherosclerotic plaque and vascular occlusion; subsequently, the disease severity of AIS patients was elevated. 10 (3) Th17 and Th1 cells were reported to promote ischemic brain injury via activating the FasL/PTPN2/TNF‐α signaling pathway. 32 Consequently, increased Th17 and Th1 cells were related to increased disease severity in AIS patients.
Lastly, this study also observed that ACC1 high (vs. low) and Th17 cell high (vs. low) were related to shortened RFS in AIS patients, while Th1 cells were not associated with RFS. Possible explanations might be as follows: (1) as discussed above, ACC1 dysregulated blood lipid, inflammatory response, and other crucial biological processes, which would cause elevated recurrence risk in AIS patients. 11 Hence, ACC1 high (vs. low) was linked with declined RFS in AIS patients. (2) Th17 cells were recognized to release proinflammatory cytokines, which aggravated neuroinflammation and accelerated AIS progression. 33 Therefore, Th17 high (vs. low) was associated with shortened RFS in AIS patients (3) Th1 cells regulated immune response whose correlation with AIS survival was relatively weak. 34 Therefore, Th1 cells were not related to RFS in AIS patients. With regard to the OS, ACC1, Th17, and Th1 cells were not related to OS in AIS patients, which might be explained as follows: During the follow‐up duration, the occurrences of death cases were limited, which weakened the statistical power.
Nonetheless, some limitations existed in the present study. Firstly, although the median follow‐up duration was 25.0 months, the correlation of ACC1 with long‐term survival in AIS patients deserved further studies. Secondly, the dynamic variation of ACC1 level during the treatment process remained unanswered. Thirdly, further studies investigating the clinical value of ACC1 in other neurological disorders were warranted. Fourthly, the previous study only suggested that both ACC1 and cell adhesion molecules participated in the formation of atherosclerosis, while their intercorrelation remained unclear. 35 Fifthly, current evidence suggested that 25%–30% of AIS patients developed immediate or delayed cognitive impairment. 36 However, the correlation of ACC1 expression with cognitive impairment remained unknown in AIS patients, and further studies were needed to investigate the issue. Sixthly, further in vivo and in vitro studies were necessary to explore the underlying mechanism of ACC1 in AIS.
Collectively, circulating ACC1 overexpression correlates with increased Th17, Th1 cells, disease severity, and shortened RFS in newly diagnosed AIS patients.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Figure S1
Table S1
Table S2
ACKNOWLEDGMENT
None.
Jiang J, Meng S, Li L, Duan X, Xu H, Li S. Correlation of acetyl‐coenzyme A carboxylase 1 with Th17 and Th1 cells, serving as a potential prognostic biomarker for acute ischemic stroke patients. J Clin Lab Anal. 2022;36:e24607. doi: 10.1002/jcla.24607
DATA AVAILABILITY STATEMENT
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Petty K, Lemkuil BP, Gierl B. Acute Ischemic Stroke. Anesthesiol Clin. 2021;39(1):113‐125. [DOI] [PubMed] [Google Scholar]
- 2. Ma Q, Li R, Wang L, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: an analysis for the global burden of disease study 2019. Lancet Public Health. 2021;6(12):e897‐e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu M, Yan M, Guo Y, et al. Acute ischemic stroke at high altitudes in China: early onset and severe manifestations. Cell. 2021;10(4):809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow YL, Teh LK, Chyi LH, Lim LF, Yee CC, Wei LK. Lipid metabolism genes in stroke pathogenesis: the atherosclerosis. Curr Pharm Des. 2020;26(34):4261‐4271. [DOI] [PubMed] [Google Scholar]
- 5. Cui P, McCullough LD, Hao J. Brain to periphery in acute ischemic stroke: mechanisms and clinical significance. Front Neuroendocrinol. 2021;63:100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuttolomondo A. Ischemic stroke pathogenesis: genetics, epigenetics and inflammation. Curr Pharm Des. 2020;26(34):4207‐4208. [DOI] [PubMed] [Google Scholar]
- 7. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208‐211. [DOI] [PubMed] [Google Scholar]
- 8. Collaborators GBDS . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanevski AN, Bjerkreim AT, Novotny V, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta Neurol Scand. 2019;140(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Duan Y, Wei H, et al. Acetyl‐CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert Opin Investig Drugs. 2019;28(10):917‐930. [DOI] [PubMed] [Google Scholar]
- 11. Gu Y, Zhang Y, Li M, et al. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front Pharmacol. 2021;12:621339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunkeler M, Hagmann A, Stuttfeld E, et al. Structural basis for regulation of human acetyl‐CoA carboxylase. Nature. 2018;558(7710):470‐474. [DOI] [PubMed] [Google Scholar]
- 13. Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole‐3‐acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. 2019;11(9):2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berod L, Friedrich C, Nandan A, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20(11):1327‐1333. [DOI] [PubMed] [Google Scholar]
- 15. Lugovaya AV, Emanuel VS, Kalinina NM, Ivanov AM, Artemova AV. Apoptosis and autophagy in the pathogenesis of acute ischemic stroke (review of literature). Klin Lab Diagn. 2020;65(7):428‐434. [DOI] [PubMed] [Google Scholar]
- 16. Nam KW, Kwon HM, Lee YS. High triglyceride‐glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci Rep. 2021;11(1):15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Zhou Y, Tang D, et al. ACC1 (Acetyl Coenzyme A Carboxylase 1) is a potential immune modulatory target of cerebral ischemic stroke. Stroke. 2019;50(7):1869‐1878. [DOI] [PubMed] [Google Scholar]
- 18. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870‐947. [DOI] [PubMed] [Google Scholar]
- 19. Mi T, Sun S, Zhang G, et al. Relationship between dyslipidemia and carotid plaques in a high‐stroke‐risk population in Shandong Province, China. Brain Behav. 2016;6(6):e00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auguet T, Berlanga A, Guiu‐Jurado E, et al. Altered fatty acid metabolism‐related gene expression in liver from morbidly obese women with non‐alcoholic fatty liver disease. Int J Mol Sci. 2014;15(12):22173‐22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lou X, Zhou X, Li H, et al. Biallelic mutations in ACACA cause a disruption in lipid homeostasis that is associated with global developmental delay, microcephaly, and dysmorphic facial features. Front Cell Dev Biol. 2021;9:618492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Q, Xu QY, Wu HM, Hua J. Effect of lipid‐induced macrophage M1/M2 polarization on lipid metabolism in hepatocytes. Zhonghua Gan Zang Bing Za Zhi. 2018;26(4):276‐281. [DOI] [PubMed] [Google Scholar]
- 23. You Q, Peng Q, Yu Z, et al. Plasma lipidomic analysis of sphingolipids in patients with large artery atherosclerosis cerebrovascular disease and cerebral small vessel disease. Biosci Rep. 2020;40(9). doi: 10.1042/BSR20201519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amelina IP, Solovieva EY. Oxidative stress and inflammation as links in a chain in patients with chronic cerebrovascular diseases. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119(4):106‐114. [DOI] [PubMed] [Google Scholar]
- 25. Mo C, Yang M, Han X, et al. Fat mass and obesity‐associated protein attenuates lipid accumulation in macrophage foam cells and alleviates atherosclerosis in apolipoprotein E‐deficient mice. J Hypertens. 2017;35(4):810‐821. [DOI] [PubMed] [Google Scholar]
- 26. Glatzel DK, Koeberle A, Pein H, et al. Acetyl‐CoA carboxylase 1 regulates endothelial cell migration by shifting the phospholipid composition. J Lipid Res. 2018;59(2):298‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Huang Y, Xu Y, et al. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2018;28(2):141‐163. [DOI] [PubMed] [Google Scholar]
- 28. Yuan S, Tang B, Zheng J, Larsson SC. Circulating lipoprotein lipids, apolipoproteins and ischemic stroke. Ann Neurol. 2020;88(6):1229‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamer F, Ulug E, Akyol A, Nergiz‐Unal R. The potential efficacy of dietary fatty acids and fructose induced inflammation and oxidative stress on the insulin signaling and fat accumulation in mice. Food Chem Toxicol. 2020;135:110914. [DOI] [PubMed] [Google Scholar]
- 30. Nikolic D, Jankovic M, Petrovic B, Novakovic I. Genetic aspects of inflammation and immune response in stroke. Int J Mol Sci. 2020;21(19):7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niu C, Chen Z, Kim KT, et al. Metformin alleviates hyperglycemia‐induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15(5):843‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng H, Zhao H, Cao X, et al. Double‐negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci USA. 2019;116(12):5558‐5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo Y, Chen X, Li D, et al. PR‐957 mediates neuroprotection by inhibiting Th17 differentiation and modulating cytokine production in a mouse model of ischaemic stroke. Clin Exp Immunol. 2018;193(2):194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ley K. Role of the adaptive immune system in atherosclerosis. Biochem Soc Trans. 2020;48(5):2273‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang C, Duan X, Sun X, et al. Protective effects of glycyrrhizic acid from edible botanical glycyrrhiza glabra against non‐alcoholic steatohepatitis in mice. Food Funct. 2016;7(9):3716‐3723. [DOI] [PubMed] [Google Scholar]
- 36. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862(5):915‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


