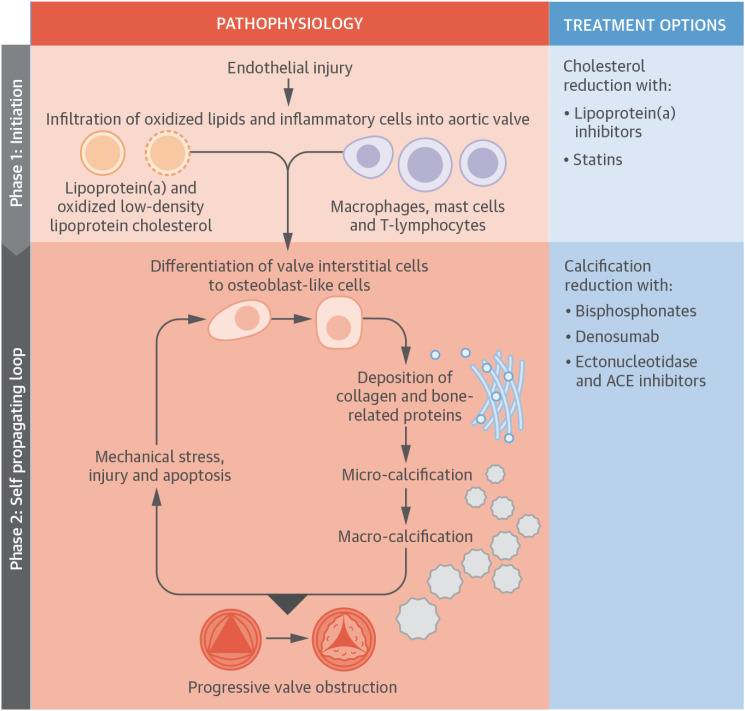
Figure 2. The pathophysiology of AS. The pathophysiology of AS is summarized. Initiation phase: endothelial injury facilitates the infiltration of oxidized lipids and inflammatory cells into the valve and the release of proinflammatory mediators. These trigger the very early stages of valve calcification. The propagation phase: these proinflammatory processes subsequently induce VICs to undergo osteogenic differentiation via several different mechanisms, including the binding of receptor activator of nuclear kappa B ligand to receptor activator of nuclear kappa B. Differentiated cells within the aortic valve first lay down a collagen matrix and other bone-related proteins causing valvular thickening and stiffening before producing calcium. Additionally, apoptotic remnants of some VICs and inflammatory cells create a nidus for apoptosis-mediated calcification. Calcification of the valve induces compliance mismatch, resulting in increased mechanical stress and injury. This results in further calcification via osteogenic differentiation and apoptosis. Hence, a self-perpetuating cycle of calcification, valve injury, apoptosis, and osteogenic activation is established that drives the propagation phase of the disease.
ACE = angiotensin-converting enzyme; AS = aortic stenosis; VIC = valvular interstitial cell.
With permission from Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol 2015;66:561-77.85)