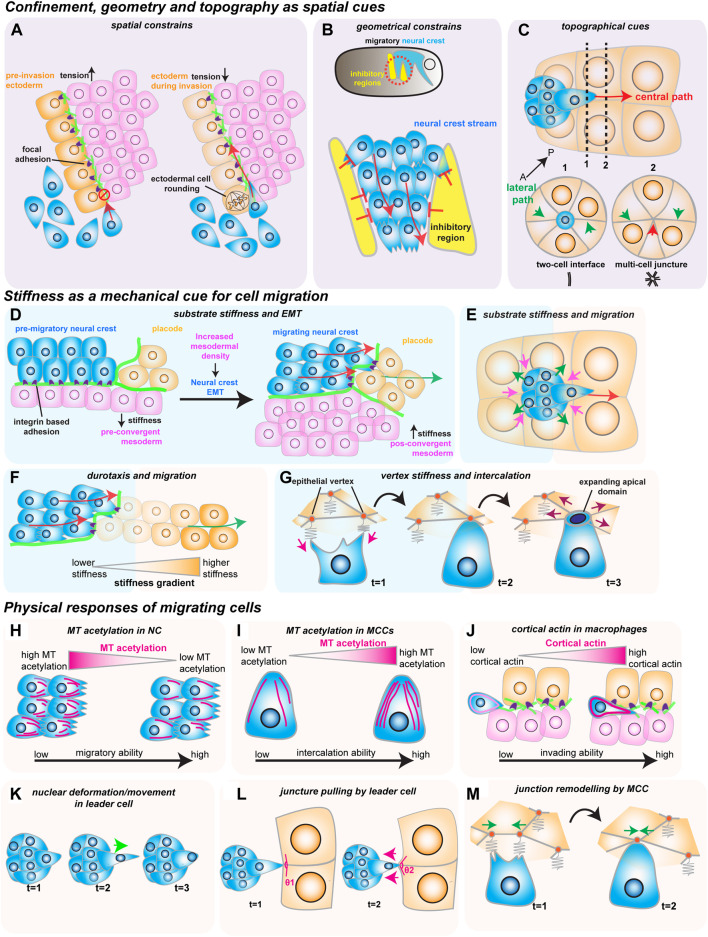
FIGURE 2.
How mechanical cues impact cell migration in vivo. The red arrows show the direction of migrating cells. (A–C) Confinement, geometry and topography as spatial cues. (A) Spatial constraints block macrophage invasion (in blue) by controlling cells’ ability to crawl through the ectoderm (in orange) and the mesoderm (in magenta). Decreasing ectodermal tension is paired with ectodermal cell rounding to promote macrophage invasion. Ectodermal cell rounding removes focal adhesions (in purple) that act as an impediment to cell movement. (B) Geometric constraints control stream formation in the neural crest (in blue). Inhibitory signals (in yellow) regulate where NC can move, stopping NC cell dispersion and promoting collective cell migration. (C) Topographic cues determine border cell migration through the central path of the egg chamber (red arrow). The central path provides more space for cluster movement which is energetically favorable, as the multi-cell junctures are easier to unzip than to the lateral paths (green arrows), which are composed of tightly juxtaposed two-cell interfaces. (D–G) Stiffness as a mechanical cue for cell migration. (D) Increase in mesoderm stiffness induces NC EMT and migration. Convergent-extension movements increase cell density and stiffness of the mesoderm (in pink), which is sensed by the pre-migratory NC (in blue) through their integrin-based adhesions (in purple). (E) Nurse cell (in orange) stiffness impacts the migration of the border cell cluster (in blue). The compressive forces from the nurse cells (magenta arrows) are counteracted by the border cells (green arrows). (F) NC cells (in blue) interact with the placodes (in orange), which causes the placodes to retreat (green arrow), generating a stiffness gradient that directs cell migration. (G) Intercalating MCCs (in blue) pull on the vertices of the neighboring goblet epithelial cells (inorange) to sense vertex stiffness (magenta arrows). The stiffer multicellular vertices act as ideal entry points into the tissue as the increased total line tension favors the opening of the MCCs apical domain (purple arrows). H-M) Physical responses of migrating cells. (H) Microtubule (MT) deacetylation decreases NC stiffness to promote NC migration (acetylated MTs in magenta). (I) MT hyperacetylation promotes MCC intercalation, possibly by increasing cell stiffness (acetylated MTs in magenta). (J) Invading macrophages generate a protective cortical actin shell that shields the nucleus from compression. (K) In the migrating border cell cluster, the leader cell protrusions are stabilized by the active movement of the nucleus to the base of the protrusion (green arrow). (L) Border cells pull on the neighboring junctures of nurse cells to sense the environment (magenta arrows). (M) Intercalating MCCs remodel the neighboring goblet cell junctions (green arrows), promoting MCC intercalation.