Abstract
The current study was designed to explore the effect of fermented camel milk, plant sterols and their combination on the blood levels of sd-LDL and atherogenicity in rats fed on high-fat-cholesterol diets (HFC). Forty male Wistar rats were distributed into five groups: Normal control (NC), Positive control (PC, HFC), plant sterol (PS, HFC containing 1% (w/w) β-sitosterol:Stigmasterols; 9:1), FM (HFC containing 4% (w/w) lyophilized fermented camel milk), and PSFM (HFC containing 1% (w/w) plant sterols +4% (w/w) lyophilized fermented camel milk). Antioxidant activity showed that β-sitosterol had the highest radical scavenging activity, followed by fermented camel milk and stigmasterol (p < 0.05). Feeding rats on HFC for 8 weeks resulted in a significant (p < 0.05) increase in blood lipids of PC group compared with NC group. Administration of PS, FM, and PSFM resulted in a significant reduction in atherogenic index (50, 24.5, and 41.5 %, p < 0.05), and sd-LDL levels (73, 45, and 59%, p < 0.05), respectively. Only the FM group showed a significant reduction in triglycerides levels of rats. Administration of PS, FM and PSFM decreased serum MDA levels significantly by 58.7, 45.4, and 69% (p < 0.05), and increased total antioxidant capacity by 35.9, 84.8, and 38.3% (p < 0.05), respectively. This is the first report to the best of our knowledge that shows fermented camel milk enriched with plant sterol could reduce atherogenesis and cardiovascular diseases activity via inhibition of the status of small dense LDL and oxidative stress.
Keywords: Cardiovascular disease, Small dense LDL, High fat cholesterol diet, Plant sterols, Fermented camel milk, Oxidative stress
Cardiovascular disease; Small dense LDL; High fat cholesterol diet; Plant sterols; Fermented camel milk; Oxidative stress.
1. Introduction
Globally, the principal causes of deaths are cardiovascular disease (CVD). It was responsible for 17 million mortalities in 2015 (Roth et al., 2017). In Saudi Arabia, CVD is a significant public health problem, as 45.7% of all deaths were linked to CVD, with an estimated 41,000 deaths each year (WHO, 2019). Furthermore, 12.5% of the total cost of healthcare in 2015 is spent on CVDs and diabetes. Both diseases cost the Saudi healthcare system about $13 billion every year (WHO, 2017).
Low-density lipoprotein (LDL) has been recognized as a leading risk factor in CVDs, and therefore it is continuously addressed in prevention of CVDs (Hoogeveen et al., 2014). However, LDL is not a single unit but is rather made up of different components (Hanig and Lauffer, 1952; Havel et al.,1955). Scientific reports have shown that small dense LDL (sd-LDL), a fraction of LDL cholesterol (LDL-C), possesses increased atherogenic potential (Hoogeveen et al.et al., 2014). The characteristic nature of sd-LDL particles makes them achieve atherogenesis easily due to their small size, extended availability in the subendothelial space (Anber et al., 1996), remaining in the blood stream longer (Kulanuwat et al., 2015), and increased chances of oxidation (Hirayama and Miida, 2012). A combination of these factors explains why sd-LDL particles of all types tend to be more atherogenic than others, and are even more precise markers for CVD than total LDL.
On the other hand, it has been found that the cardiovascular risk can be mitigated significantly when people make adjustments to their diet (Köhler et al., 2017). In this regard, studies for over 6 decades show the lowering effect of phytosterols on LDL-C (Peterson, 1958; Pollak, 1953). Phytosterols are compounds that are naturally found in plants and can be found in a wide variety of plant-based food, vegetable oils and grain products (Salehi et al., 2021). A meta-analysis of 124 randomized controlled trials concluded that a phytosterol dose of 0.6–3.3 g daily gradually reduced the level of LDL-C by 6–12% where plant sterols and stanols show similar effects. the mean phytosterol dose was 2.1 g/day (doses ranged from 0.2 to 9.0 g/day) (Ras et al., 2014). Other studies showed that plasma total cholesterol and LDL-C can be reduced with plant sterol in hamsters fed high-fat-diet (Meijer et al., 2003; Piironen et al., 2000).
The main mechanism that allows phytosterols to cause a reduction in cholesterol is their inhibitory action to intestinal cholesterol absorption, as both plant sterols and cholesterol compete for absorption in the intestine (Trautwein et al., 2003). A study conducted by some scientists discovered that plant sterols can decrease the occurrence of cardiovascular diseases by mechanisms different from the cholesterol-lowering effect (Oliveira Godoy Ilha et al., 2020). The study demonstrated that plant sterol causes a marked decrease in plasma levels of endothelin-1 and could improve endothelial function, apart from reducing plasma levels of cholesterol and LDL-Cholesterol.
Various food products have been fortified with phytosterol for individuals who desire to reduce the level of LDL-cholesterol concentration in their blood (Clement et al., 2010). However, one common and readily available food carrier of phytosterols is dairy food (Cheung et al., 2017). Different experiments with different study designs have been undertaken to study how plant sterol/stanols-fortified milk affects the plasma lipid profile. Human studies suggest that LDL-C concentration and cholesterol metabolism markers generally improves with the intake of phytosterol-rich milk (Andrade et al., 2015; Bañuls et al., 2010; Ribas et al., 2017). A randomized controlled trial demonstrated that subjects who were given powder milk fortified with 1.5 g of phytosterols daily over three weeks showed a marked decrease in their LDL-cholesterol levels, without the use of cholesterol-lowering drugs or diabetes mellitus. The study, conducted in Southern China, was a double-blind trial with 221 subjects. Compared to the control group, the group receiving the fortified milk showed a difference of (9.5 ± 2.0%; P < 0.0001) in their level of LDL-cholesterol (Cheung et al., 2017).
In animal models, a study carried out in hamsters fed high-fat-high-cholesterol diet showed a reduced level of serum total cholesterol, LDL-cholesterol, and LDL-cholesterol/HDL-cholesterol ratio, as well as hepatic lipid levels when administered with phytosterol-containing lactic-fermented milk powder (Chien et al., 2010). This milk powder, containing 0.74% and 1.85% phytosterol/phytostanol, also caused an increase in fecal cholesterol and phytosterol levels, due to the inhibitory absorption of the intestines of dietary cholesterol, as well as the re-absorption of biliary cholesterol.
Milk is important in nutrition, as it contains a unique composition of proteins, vitamins, carbohydrates, and minerals (Hussain et al., 2021). Camel milk, a famous dairy food in many parts of the word, particularly in arid and semi-arid regions (Ashraf et al., 2021). Camel milk's therapeutic properties against hypocholesterolemia were studied in several animal models. However, the way these properties work is not fully understood. Some studies found that bioactive peptides in camel milk, obtained from milk proteins, interact with cholesterol and lowered its absorption (Li and Papadopoulos, 1998). Other studies found that orotic acid in camel milk may be reducing agent of cholesterol levels either in humans (Buonopane et al., 1992) or in rats (Rao et al., 1981).
Therefore, this study aims to evaluate the effects of phytosterol and fermented camel milk combination on the blood levels of sd-LDL particles in rats fed on high-fat-cholesterol diet.
2. Material and methods
2.1. Material
Camel milk used in this study was collected from the experimental station of animal production at Qassim University. Yoghurt DVS culture (YF-L811) - consists of Lactobacillus bulgaricus and Streptococcus thermophilus - was obtained from Chr. Hansen's (Denmark). Plant sterols (β-sitosterol and Stigmasterols) were purchased from HIMEDIA (HiMedia Laboratories Pvt. Ltd., India). The components of the experimental diets were locally purchased. All chemicals used in the study were of analytical grade.
2.2. Methods
2.2.1. Preparation of yoghurt
Yoghurt was prepared as described by (Tamime and Robinson, 2007) in the food processing pilot plant of the Food Science and Human Nutrition Department, Qassim University, Saudi Arabia. Camel milk composition was measured using Funke Gerber Lactostar milk analyzer (Waring Laboratory Products, Canada). Milk fat was removed from Camel milk by using milk separator. Then, camel milk (3.1% protein, 0.1% fat, 4.9% lactose and 0.73 salts) was heated in a water bath at 85 °C for 5 min, cooled to approximately 43 °C., inoculated with a starter culture (YF-L811, 3% v:v), and incubated at 43 °C until the milk pH-value reached 4.6. The final fermented milk then stored at -18 °C, and then freeze-dried (Laboratory freeze-dryer, Martin Christ Gefriertrocknungsanlagen GmbH, Germany).
2.2.2. Experimental design
2.2.2.1. Animals & diets
Male Wistar rats (5–7 weeks old, body weight of 150–200 g) were purchased from the experimental animal unit, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Rats were housed in laboratory polypropylene rats’ cages (4 per cage/2 cages per group) under standard laboratory conditions of 22–23 °C, 12 h light/dark cycle, under relative humidity of 50% ± 5 in experimental animal house at the Department of Food Science and Human Nutrition, College of Agriculture and Vet. Med., Qassim University. The experimental protocol of this study was approved by the Qassim University Committee for Scientific Research Ethics (Approval #21-5-14).
The high-fat-cholesterol (HFC) diet was selected as it has been shown to induce similar metabolic responses and atherosclerosis development in animals compared to humans (Watanabe et al., 2017). The composition of standard basal diet is according to AIN-93 guidelines (Reeves et al., 1993) The HFC diet was formulated according to the standard formula of Research Diets Inc. (Hyeran, 2017) and as described by (Naito et al., 2019). All experimental HFC diets were formulated to contain 1% cholesterol and to represent 20% and 45% of energy from protein and fat, respectively, by the addition of casein and coconut oil.
2.2.2.2. Experimental groups
Forty rats were distributed into five groups (n = 8 for each group) as follow:
NC: Normal control group (rats were fed on standard basal diet).
PC: Positive control group (rats were fed on HFC diet).
PS: Rats were fed on HFC diet containing 1% (w/w) plant sterols consists pf β-sitosterol and Stigmasterols (9:1).
FM: Rats were fed on HFC diet containing 4% (w/w) lyophilized fermented camel milk.
PSFM: Rats were fed on HFC diet containing 1% (w/w) plant sterols consists pf β-sitosterol and Stigmasterols (9:1) + 4% (w/w) lyophilized fermented camel milk.
The detailed compositions of the experimental diets are shown in Table 1. All groups were fed on experimental diets for 8 successive weeks. The dose of plant sterols (1% in diet) was chosen according to the (Carr et al., 2002). The dose of fermented camel milk (4% in diet) was selected depends on a previous report (Yahya et al., 2018).
Table 1.
Composition of the experimental diets.
| Components (g/kg diet) | Groups |
||||
|---|---|---|---|---|---|
| NC | PC | PS | FM | FMPS | |
| LFCM∗ | - | - | - | 40 | 40 |
| βSitosterol | - | - | 9.2 | - | 9.2 |
| Stigmasterol | - | - | 0.8 | - | 0.8 |
| Casein | 200 | 220 | 220 | 200 | 200 |
| Coconut oil | - | 130 | 130 | 130 | 130 |
| Cholesterol | - | 10 | 10 | 10 | 10 |
| Corn oil | 50 | 50 | 40 | 50 | 39 |
| α-corn starch | 150 | 100 | 100 | 100 | 100 |
| Sucrose | 400 | 240 | 240 | 220 | 220 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| AIN-76 Minerals Mix | 35 | 35 | 35 | 35 | 35 |
| AIN-76 Vitamins Mix | 10 | 10 | 10 | 10 | 10 |
| D,L-methionine | 3 | 3 | 3 | 3 | 3 |
| Energy Density (kcal/g diet) |
3.75 |
4.55 |
4.55 |
4.55 |
4.55 |
| Energy (kcal, %) | |||||
| Protein | 19.5 | 18.7 | 18.7 | 18.5 | 18.5 |
| Carbohydrate | 66.5 | 36.3 | 36.3 | 36.5 | 36.5 |
| Fat | 14.0 | 45.0 | 45.0 | 45.0 | 45.0 |
∗ Lyophilized fermented camel milk.
NC: Normal control, PC: Positive control, PS: Plant Sterols, FM: Fermented camel milk, PSFM: Fermented camel milk enriched with plant sterols.
Food consumption and body weight for each animal were recorded at the beginning of experimental period and at 7 days intervals. Initial and final body weights were recorded and body weight gain was calculated. At the end of the experiment and after an overnight fasting (12 h), rats were sacrificed by decapitation, blood samples were collected in plane tubes and centrifuged at 3000 rpm to harvest the serum which was stored at (–20 °C) for biochemical analysis.
2.2.3. Determination of serum lipid profile
Commercial kits were utilized for measurement of Cholesterol (Allain et al., 1974), and Triglyceride (Stein and Myers, 1995), HDL (Kostner et al., 1979). The concentration of LDL was calculated according to the formula of (Friedewald et al., 1972) (LDL = Total cholesterol – HDL cholesterol – Triglyceride/5).
2.2.4. Determination of atherogenic index of plasma (AIP)
Atherogenic Index of Plasma (AIP) values were calculated according to formula of (Dobiašova, 2004).
| AIP = log TG/HDL-c |
2.2.5. Determination of sd-LDL particles
Small and dense LDL particles were calculated according to formula of (Srisawasdi et al., 2011) small, dense particles in mg/dL = 0.580 (non–HDL-C) + 0.407 (direct LDL-C) – 0.719 (calculated LDL-C) – 12.05, where calculated LDL-C = Total cholesterol – HDL-C – (Triglycerides/5) was calculated.
2.2.6. Malondialdehyde (MDA)
The collected serum was used to determine lipid peroxides as malondialdehyde (MDA) according to the method described by (Namıduru et al., 2011).
2.2.7. DPPH radical-scavenging activity
DPPH radical scavenging activity of camel milk and plant sterols were determined according to the method of (Musa et al., 2011).
2.2.8. ABTS radical scavenging assay
Free radical scavenging activity of camel milk and plant sterols was determined according to (Re et al., 1999).
2.2.9. Determination of glutation peroxidase (GPx) activity
Commercial reagents were purchased from Nanjing Jiancheng Bioengineering Company, Nanjing, China to determine the activity of glutathione peroxidase (GPx) according to the method of (Paglia and Valentine, 1967).
2.2.10. Determination of total antioxidant capacity
Total antioxidant capacity was determined in serum according to (Koracevic et al., 2001).
2.2.11. Statistical analysis
Results were presented as means ± SD and the data were subjected to one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons by using SPSS ver. 22.0 statistical package software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Differences were considered to be statistically significant at P < 0.05.
3. Results
3.1. Antioxidant activity of β-sitosterol, stigmasterol and camel milk
Results from (Table 2) show that β-sitosterol had the highest (77.6%) DPPH radical scavenging activity, followed by camel milk (67%) and stigmasterol (60.9%). In the ABTS assay, β-sitosterol again had the highest (84.4%) scavenging activity of all the ingredients, followed by stigmasterol (71.6%) and camel milk (51.6%). A significant difference (P < 0.05) between all the components was observed.
Table 2.
Radical-scavenging activity (DPPH and ABTS) of β-sitosterol, Stigmasterol and Fermented camel milk.
| Test | β-sitosterol | Stigmasterol | Fermented camel milk |
|---|---|---|---|
| DPPH | 77.64 ± 2.200a | 60.98 ± 1.692c | 67.01 ± .861b |
| ABTS | 84.47 ± .438a | 71.68 ± .469b | 51.62 ± 1.620c |
Values in the same row bearing different superscript letters (a to c) are different (P < 0.05).
Values are means ± SD. (n = 3).
3.2. Body and liver weights
There were no significant differences (P < 0.05) observed in body weights between experimental groups (Table 3). All rats gained weight comparably regardless of treatments. Liver weight was decreased significantly in all treated groups as compared with the PC group (P < 0.05). As for the liver weight relative to body weight (liver index), PS and PSFM produced a slight reduction, while FM remarkably decreased the relative liver weight by 13.7% (P < 0.05) compared with PC group rats (Table 2).
Table 3.
Some growth parameters of rats fed on high -fat -cholesterol diet containing plant sterols, fermented camel milk and their combination.
| Groups | Initial body weight (g) | Final body weight (g) | Weight gain (g) | Liver weight (g) | Liver Index (% of FBW) |
|---|---|---|---|---|---|
| NC | 286.3 ± 20.8a | 346.7 ± 36.6a | 67.0 ± 37.5a | 9.7 ± 1.4b | 2.7 ± 0.2b |
| PC | 286.5 ± 21.1a | 371.9 ± 30.1a | 78.3 ± 30.6a | 11.8 ± 0.5a | 3.1 ± 0.08a |
| FM | 277.0 ± 18.2a | 339.3 ± 18.9a | 57.9 ± 21.3a | 9.4 ± 0.8b | 2.7 ± 0.3b |
| PS | 277.7 ± 18.1a | 353.4 ± 31.0a | 75.8 ± 28.8a | 9.7 ± 0.9b | 2.8 ± 0.2ab |
| PSFM | 277.2 ± 17.8a | 358.6 ± 25.1a | 81.5 ± 37.7a | 9.8 ± 0.9b | 2.7 ± 0.4ab |
Values are means ± SD. (n = 8).
Values in the same column bearing different superscript letters are different (P < 0.05).
NC: Normal control, PC: Positive control, PS: Plant Sterols, FM: Fermented camel milk, PSFM: Fermented camel milk enriched with plant sterols.
3.3. AIP and sd-LDL values
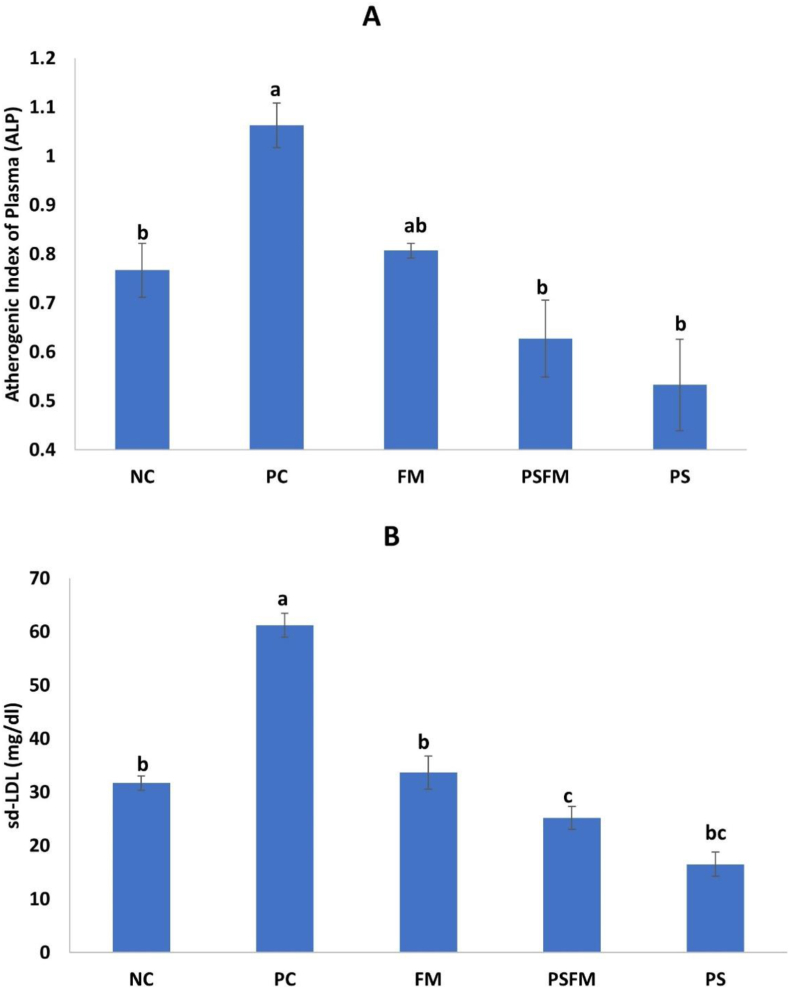
Eight weeks after feeding the HCF diet, a significant increase was observed in the AIP and sd-LDL in PC group compared to the NC group (P < 0.05). After administration of PS, FM, and PSFM, the AIP values was decreased by 50% (P < 0.05), 24.5% (P < 0.05), and 41.5 % (P < 0.05) when compared to the PC group (Figure 1A). Similarly, PS, FM, and PSFM significantly reduced sd-LDL levels by 73 % (P < 0.05), 45 % (P < 0.05), and 59 % (P < 0.05), respectively (Figure 1B).
Figure 1.
Changes in atherogenic index of plasma (A), and sd-LDL levels (B) in rats fed on high fat cholesterol diet containing plant sterols, fermented camel milk and their combination. NC: normal control, PC: positive control, PS: Plant Sterols, FM: Fermented camel milk, PSFM: Fermented camel milk enriched with plant sterols. Different letters indicate a significant difference between groups (P < 0.05).
3.4. Lipid profile serum levels
The results show that feeding rats a high-fat-cholesterol diet for 8 weeks resulted in a significant (P < 0.05) increase in LDL, TC, and TG levels and slightly lower HDL levels in the PC group compared to the NC group. However, after administration of PS, FM, and PSFM, the LDL and TC levels decreased significantly.
The PS and PSFM groups had a slight but not significant decrease in serum TG, but the FM groups had a significant decrease in comparison with the PC group. Moreover, FM and PSFM produced a slight increase in the HDL serum level, whereas PS alone remarkably increased the HDL serum level compared with PC group rats (Table 4).
Table 4.
Serum Lipid profile of rats fed on high -fat -cholesterol diet containing plant sterols, fermented camel milk and their combination.
| Groups | LDL | HDL | TC | TG |
|---|---|---|---|---|
| NC | 85.47 ± 12.20b | 45.88 ± 23.19ab | 160.20 ± 19.33b | 195.76 ± 7.90b |
| PC | 194.3 ± 24.45a | 24.39 ± 10.56b | 252.53 ± 18.63a | 228.72 ± 16.48a |
| FM | 91.33 ± 36.22b | 30.90 ± 1.90ab | 157.04 ± 20.42b | 203.52 ± 12.40b |
| PS | 86.73 ± 37.22b | 62.66 ± 19.49a | 111.87 ± 26.25c | 207.07 ± 12.17ab |
| PSFM | 54.23 ± 16.78b | 45.28 ± 11.58ab | 154.28 ± 18.16b | 207.92 ± 2.63ab |
Values are means ± SD. (n = 8).
Values in the same column bearing different superscript letters are different (P < 0.05).
LDL: low-density lipoprotein cholesterol, HDL: High-density lipoprotein cholesterol, TC: Total cholesterol, TG: Triacylglycerol.
NC: Normal control, PC: Positive control, PS: Plant Sterols, FM: Fermented camel milk, PSFM: Fermented camel milk enriched with plant sterols.
3.5. Oxidative stress parameters (GSH-Px activities, total antioxidant capacity, and MDA level)
Statistical analyses indicated significantly higher MDA and lower GSH-Px and TAC levels in the PC group compared with normal control (Table 4). However, PS and FM groups decreased serum MDA levels by 58.7% (P < 0.05) and 45.4 % (P < 0.05) respectively, whereas PSFM group decreased it by 69 % (P < 0.05). PS, FM and PSFM groups significantly increased the TAC level by 35.9% (P < 0.05), 84.8 % (P < 0.05) and 38.3 % (P < 0.05), respectively, in comparison with that of PC controls. PS and PSFM groups produced a slight but not significant increase in serum GSH-Px, whereas FM groups possessed a significant increase, in comparison with that of PC group (Table 5).
Table 5.
Oxidative stress parameters of rats fed on high -fat -cholesterol diet containing plant sterols, fermented camel milk or their combination.
| Groups | MDA | GSH-Px | TAC |
|---|---|---|---|
| NC | 2.15 ± 0.91b | 27.56 ± 12.15a | 6.33 ± 0.38a |
| PC | 6.09 ± 0.51a | 7.62 ± 2.37b | 3.23 ± 0.39c |
| FM | 3.32 ± 1.24b | 25.46 ± 7.89a | 5.97 ± 1.03a |
| PS | 2.52 ± 0.74b | 17.15 ± 5.22ab | 4.39 ± 0.451b |
| PSFM | 1.88 ± 0.96b | 14.07 ± 2.28b | 4.47 ± 0.65b |
Values are means ± SD. (n = 8).
Values in the same column bearing different superscript letters are different (P < 0.05).
MDA: Malondialdehyde, GSH-Px: Glutathione Peroxidase, TAC: Total Antioxidant Capacity.
NC: Normal control, PC: Positive control, PS: Plant Sterols, FM: Fermented camel milk, PSFM: Fermented camel milk enriched with plant sterols.
4. Discussion
Many researchers have been able to demonstrate that phytosterols indeed have a lipid-lowering effect (Bañuls et al., 2010; Racette et al., 2010; Ribas et al., 2017). In addition, studies have also reported that camel milk has numerous health benefits, one of which is a significant effect on serum lipid profile (Korish and Arafah, 2013; Yahya et al., 2018). In this current study, both PS and FM elicited diverse lipid-lowering abilities, whether when used singularly or combined with each other.
According to the results, administration of PS and FM alone or in combination improved serum TC levels. These findings are consistent with those of (Chien et al., 2010) who found that the TC levels in the serum of hamster fed phytosterol-containing lactic-fermented milk powder inoculated with probiotic mixture (L. acidophilus, B. lactis, S. thermophilus and L. bulgaricus) was lower than the TC level of control group rats.
Serum TG levels were also reduced by FM. These results agree with the results from a study by (Yahya et al., 2018) who observed that the consumption of fermented skim camel milk made with each of the L. helveticus and S. thermophilus strains lowered the concentration of TG in rats fed a cholesterol-enriched diet. Additionally, in the PS and PSFM groups, there was a reduction in TG levels, but such changes had little statistical impact. These slight decreases are consistent with results from an analysis of 12 randomized controlled trials which demonstrated that a daily phytosterol intake of about 2g can lower TG levels insignificantly. However, they suggested that the changes in TG construction appear to depend on the baseline TG level (Demonty et al., 2013).
In the present study, there was an increase in HDL levels in the PS group and a slight increase in the FM and PSFM groups compared with the PC group. Plant sterols usually have a small or no effect on HDL cholesterol concentrations (Gylling and Miettinen, 1994; Katan et al., 2003; Moreau et al., 2002). However, a rise in HDL was observed in a study employing orange juice with plant sterols (Devaraj et al., 2006). For camel milk, various levels of HDL-improving effects were observed in some previous studies (Ali et al., 2013; Yahya et al., 2018).
As for low-density lipoprotein, plant sterols have a significant impact on it. This is further corroborated by clinical trials, which have continually proven that a daily plant sterol intake of 2–3g can reduce LDL levels by 4.1–15% (Abumweis et al., 2008; Malinowski and Gehret, 2010). Furthermore, a study by (Ali et al., 2013) showed that rats who were given fermented camels milk possessed lower LDL levels, compared to their counterparts who were given cow's milk. Similarly, lactic acid bacteria-fermented milk proved to be particularly effective in lowering the concentration of LDL in the blood of hamsters (Chiu et al., 2006). This is consistent with our results that showed that PS and FM considerably lower serum LDL levels. Furthermore, when PS and FM were given together, there was a slight additive effect of PS and FM on LDL. However, as mentioned earlier, when LDL particles are small and dense, they will have greater atherogenicity, due to the fact that they penetrate faster into the walls of arteries, have a lower binding capacity as regarding LDL receptors and less resistance to oxidative stress among various other features (Manabe et al., 2015). Moreover, previously conducted studies have found a strong link between sd-LDL particle concentration and CVD events (Nakou et al., 2008). Therefore, the occurrence of cardiovascular diseases and atherosclerosis should likely be prevented by a marked reduction in the concentration of sdLDL particles. Based on this evidence, our study is able to establish one key point that plant sterol and camel milk, either alone or in combination, lower sdLDL proportions. The results also display that serum sd-LDL-lowering effect of FM was further strengthened when combined with PS. In agreement with our results, several studies have already shown the benefits of regular consumption of foods containing phytosterols in decreasing sd-LDL-C concentrations (Garoufi et al., 2014; Sialvera et al., 2012). A reduction in sd-LDL particles can be theorized by an improvement in oxidative status in the body. Less oxidative stress might result in a reduced production of sd-LDL simply because small LDL are found to increase almost always in periods of high oxidative stress (Kotani et al., 2012). This improvement in oxidative status could be achieved by ingredients with antioxidant properties, which is what our components possess, as shown in Table 1, where the antioxidant activity of plant sterol and camel milk was evaluated based on the radical scavenging activities using both methods, DPPH and ABST.
We also examined atherogenic indices of plasma since it is a strong predictor of atherosclerosis (Niroumand et al., 2015) and studies have shown that it can predict cardiovascular risk and treatment efficacy (Bhardwaj et al., 2013). Our data indicated that the PSFM was more efficient in lowering AIP than the FM alone. The FM showed a slight decrease in AIP, while the PS and PSFM showed an obvious decrease in AIP.
Atherosclerosis is an inflammatory process primarily precipitated by oxidative stress. Studies show that atherosclerosis and hypercholesteremia are intertwined, hypercholesteremia being a dominant risk factor for oxidative stress. The condition does this by encouraging the formation of free oxygen radicals, which may lead to the formation of malondialdehyde (MDA) caused by lipid peroxidation (Dikshit et al., 2016; Lovrić et al., 2008). MDA is an important product of lipid hydroperoxides degradation and hence, makes it a suitable marker for checking the degree of lipid peroxidation (Del Rio et al., 2005). This study showed that MDA levels were high in animals who were fed a high-cholesterol diet, indicating a decrease in antioxidant status, which is consistent with other studies (Korish and Arafah, 2013; Küskü-Kiraz et al., 2010). A decrease in MDA occurred when PS and FM alone or in combination were co-administered with high fat cholesterol. Data from our treatment groups suggested that both substances can effectively reduce lipid peroxidation and enhance antioxidant status. Moreover, present study also showed that PS, FM and PSFM groups not only decreased the level of MDA but also increased the level of TAC in serum of rats. This is consistent with the finding in other antioxidants, such as omega-3 fatty acids (Ali and Rifaai, 2019) and Cinnamon Polyphenol Extract (Tuzcu et al., 2017).
We also investigated the effect of PS and FM alone or in combination on serum antioxidant enzyme GSH-Px activity. There was a decrease in GSH-Px activity in rats fed on HFC diet compared to normal control group. The reduction in GHS-Px activity could be caused by the role it plays in the neutralization of free radicals generated by hyperlipidemia (Jiangwei et al., 2011). FM group showed an increase in GSH-Px activity. The result was supported by a study (Zuberu et al., 2017) which demonstrated that camel milk raises the level GSH-Px in the blood, probably as a result of the milk's magnesium content. Magnesium, along with glycine, γ-glutamyl cysteine, and ATP is a key ingredient in the biosynthesis of glutathione synthetase and thus glutathione. PS and PSFM groups showed slight increase in GSH-Px activity. Previous in vitro studies have also shown that PS can enhance GSH-Px antioxidant enzyme activity (Vivancos and Moreno, 2005) and in vivo studies (Yuan et al., 2021). The results above showed that PSFM might be a useful dietary supplement against excessive oxidative stress and could improve antioxidant status.
5. Conclusion
The results of this study show that the combination of PS and FM could be useful in the development of effective natural products for improving oxidative state and lowering blood lipids, particularly sd-LDL, which has been linked to significant reductions in atherogenesis and CVD events. However, further research is needed to understand the exact mechanisms of action and to what extent this mixture of plant sterols and camel milk can influence the sd-LDL level. Further studies on the expression of key genes are clearly needed to better understand the effects on the molecular level.
Declarations
Author contribution statement
Alamro, S.: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Al Abdulmonem, W.: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data; Wrote the paper.
Althwab, S.; Hamad, E.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Allemailem, K.: Contributed reagents, materials, analysis tools or data; Wrote the paper; Analyzed and interpreted the data.
Alarifi, S.: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by Qassim University, represented by the Deanship of Scientific Research under the number (10341-cavm-2020-1-3-I) during the academic year 1441 AH/2020 AD.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the financial support for this research under the number (10341-cavm-2020-1-3-I) during the academic year 1441 AH/2020 AD.
References
- Abumweis S., Barake R., Jones P. Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr. Res. 2008;52(1):1811. doi: 10.3402/fnr.v52i0.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.A., Alyan A.A., Bahobail A.S. Effect of fermented camel milk and cow milk containing (bifidobacteria) enriched diet in rats fed on cholesterol level. Agric. Sci. Res. J. 2013;3(11):342–346. [Google Scholar]
- Ali F.F., Rifaai R.A. Preventive effect of omega-3 fatty acids in a rat model of stress-induced liver injury. J. Cell. Physiol. 2019;234(7):11960–11968. doi: 10.1002/jcp.27848. [DOI] [PubMed] [Google Scholar]
- Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- Anber V., Griffin B.A., McConnell M., Packard C.J., Shepherd J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis. 1996;124(2):261–271. doi: 10.1016/0021-9150(96)05842-x. [DOI] [PubMed] [Google Scholar]
- Andrade I., Santos L., Ramos F. Add-on” effect of phytosterols-enriched fermented milk on lipids and markers of cholesterol metabolism in statin-treated elderly patients. Steroids. 2015;99:293–298. doi: 10.1016/j.steroids.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Ashraf A., Mudgil P., Palakkott A., Iratni R., Gan C., Maqsood S., Ayoub M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021;104(1):61–77. doi: 10.3168/jds.2020-18627. [DOI] [PubMed] [Google Scholar]
- Bañuls C., Martínez-Triguero M.L., López-Ruiz A., Morillas C., Lacomba R., Víctor V.M., Rocha M., Hernández-Mijares A. Evaluation of cardiovascular risk and oxidative stress parameters in hypercholesterolemic subjects on a standard healthy diet including low-fat milk enriched with plant sterols. J. Nutr. Biochem. 2010;21(9):881–886. doi: 10.1016/j.jnutbio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S., Bhattacharjee J., Bhatnagar M.K., Tyagi S., Delhi N. Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int. J. Pharm. Biol. Sci. 2013;3(3):359–364. [Google Scholar]
- Buonopane G.J., Kilara A., Smith J.S., McCarthy R.D. Effect of skim milk supplementation on blood cholesterol concentration, blood pressure, and triglycerides in a free-living human population. J. Am. Coll. Nutr. 1992;11(1):56–67. doi: 10.1080/07315724.1992.10718197. [DOI] [PubMed] [Google Scholar]
- Carr T.P., Cornelison R.M., Illston B.J., Stuefer-Powell C.L., Gallaher D.D. Plant sterols alter bile acid metabolism and reduce cholesterol absorption in hamsters fed a beef-based diet. Nutr. Res. 2002;22(6):745–754. [Google Scholar]
- Cheung C., Ho D.K., Sing C., Tsoi M., Cheng V.K., Lee G.K., Ho Y., Cheung B.M. Randomized controlled trial of the effect of phytosterols-enriched low-fat milk on lipid profile in Chinese. Sci. Rep. 2017;7(1):1–6. doi: 10.1038/srep41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y.L., Wu L.Y., Lee T.C., Hwang L.S. Cholesterol-lowering effect of phytosterol-containing lactic-fermented milk powder in hamsters. Food Chem. 2010;119(3):1121–1126. [Google Scholar]
- Chiu C., Lu T., Tseng Y., Pan T. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2006;71(2):238–245. doi: 10.1007/s00253-005-0145-0. [DOI] [PubMed] [Google Scholar]
- Clement L.M., Hansen S.L., Costin C.D., Perri G.L. Quantitation of sterols and steryl esters in fortified foods and beverages by GC/FID. J. Am. Oil Chem. Soc. 2010;87(9):973–980. [Google Scholar]
- Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metabol. Cardiovasc. Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Demonty I., Ras R.T., van der Knaap H., Meijer L., Zock P.L., Geleijnse J.M., Trautwein E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomized controlled trials. Eur. J. Nutr. 2013;52(1):153–160. doi: 10.1007/s00394-011-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S., Autret B.C., Jialal I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am. J. Clin. Nutr. 2006;84(4):756–761. doi: 10.1093/ajcn/84.4.756. [DOI] [PubMed] [Google Scholar]
- Dikshit P., Tyagi M.K., Shukla K., Gambhir J.K., Shukla R. Antihypercholesterolemic and antioxidant effect of sterol rich methanol extract of stem of Musa sapientum (banana) in cholesterol fed wistar rats. J. Food Sci. Technol. 2016;53(3):1690–1697. doi: 10.1007/s13197-015-2096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobiašova M. Atherogenic index of plasma [log (triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin. Chem. 2004;50(7):1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Garoufi A., Vorre S., Soldatou A., Tsentidis C., Kossiva L., Drakatos A., Marmarinos A., Gourgiotis D. Plant sterols–enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: a prospective study. Ital. J. Pediatr. 2014;40(1):1–6. doi: 10.1186/1824-7288-40-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylling H., Miettinen T.A. Serum cholesterol and cholesterol and lipoprotein metabolism in hypercholesterolaemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia. 1994;37(8):773–780. doi: 10.1007/BF00404334. [DOI] [PubMed] [Google Scholar]
- Hanig M., Lauffer M.A. Ultracentrifugal studies of lipoproteins in diabetic sera. Diabetes. 1952;1(6):447–448. doi: 10.2337/diab.1.6.447. [DOI] [PubMed] [Google Scholar]
- Havel R.J., Eder H.A., Bragdon J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama S., Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin. Chim. Acta. 2012;414:215–224. doi: 10.1016/j.cca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Hoogeveen R.C., Gaubatz J.W., Sun W., Dodge R.C., Crosby J.R., Jiang J., Couper D., Virani S.S., Kathiresan S., Boerwinkle E. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain H., Wattoo F.H., Wattoo M.H.S., Gulfraz M., Masud T., Shah I., Ali S., Alavi S.E. Camel milk as an alternative treatment regimen for diabetes therapy. Food Sci. Nutr. 2021;9(3):1347–1356. doi: 10.1002/fsn3.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyeran J. Research Diets, Inc.; New Brunswick, NJ: 2017. High-Fat Diets for Diet-Induced Obesity (DIO) Models. 2017- Brief Scientific Literature Review”. Research Diets (2017) [Google Scholar]
- Jiangwei M.A., Zengyong Q., Xia X. Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J. Med. Plants Res. 2011;5(5):855–858. [Google Scholar]
- Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R., Participants S.W. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003;78(8):965–978. doi: 10.4065/78.8.965. Paper presented at the. [DOI] [PubMed] [Google Scholar]
- Köhler J., Teupser D., Elsässer A., Weingärtner O. Plant sterol enriched functional food and atherosclerosis. Br. J. Pharmacol. 2017;174(11):1281–1289. doi: 10.1111/bph.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54(5):356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korish A.A., Arafah M.M. Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease. BMC Compl. Alternative Med. 2013;13(1):1–12. doi: 10.1186/1472-6882-13-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostner G.M., Avogaro P., Bon G.B., Cazzolato G., Quinci G.B. Determination of high-density lipoproteins: screening methods compared. Clin. Chem. 1979;25(6):939–942. [PubMed] [Google Scholar]
- Kotani K., Tsuzaki K., Taniguchi N., Sakane N. LDL particle size and reactive oxygen metabolites in dyslipidemic patients. Int. J. Prev. Med. 2012;3(3):160. [PMC free article] [PubMed] [Google Scholar]
- Kulanuwat S., Tungtrongchitr R., Billington D., Davies I.G. Prevalence of plasma small dense LDL is increased in obesity in a Thai population. Lipids Health Dis. 2015;14(1):30. doi: 10.1186/s12944-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küskü-Kiraz Z., Mehmetçik G., Doǧru-Abbasoǧlu S., Uysal M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytother Res.: Int. J. Devot. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010;24(4):565–570. doi: 10.1002/ptr.2985. [DOI] [PubMed] [Google Scholar]
- Li H., Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139(12):4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Lovrić J., Mesić M., Macan M., Koprivanac M., Kelava M., Bradamante V. Measurement of malondialdehyde (MDA) level in rat plasma after simvastatin treatment using two different analytical methods. Period. Biol. 2008;110(1):63–68. [Google Scholar]
- Malinowski J.M., Gehret M.M. Phytosterols for dyslipidemia. Am. J. Health Syst. Pharm. 2010;67(14):1165–1173. doi: 10.2146/ajhp090427. [DOI] [PubMed] [Google Scholar]
- Manabe Y., Morihara R., Matsuzono K., Nakano Y., Takahashi Y., Narai H., Omori N., Abe K. Estimation of the presence of small dense lipoprotein cholesterol in acute ischemic stroke. Neurol. Int. 2015;7(1) doi: 10.4081/ni.2015.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer G.W., Bressers M.A., Arjan de Groot W., Rudrum M. Effect of struture and form on the ability of plant sterols to inhibit cholesterol absorption in hamsters. Lipids. 2003;38(7):713–721. doi: 10.1007/s11745-003-1119-4. [DOI] [PubMed] [Google Scholar]
- Moreau R.A., Whitaker B.D., Hicks K.B. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002;41(6):457–500. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Musa K.H., Abdullah A., Jusoh K., Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Anal. Methods. 2011;4(1):100–107. [Google Scholar]
- Naito H., Yoshikawa-Bando Y., Yuan Y., Hashimoto S., Kitamori K., Yatsuya H., Nakajima T. High-fat and high-cholesterol diet decreases phosphorylated inositol-requiring kinase-1 and inhibits autophagy process in rat liver. Sci. Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-48973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakou E.S., Filippatos T.D., Georgoula M., Kiortsis D.N., Tselepis A.D., Mikhailidis D.P., Elisaf M.S. The effect of orlistat and ezetimibe, alone or in combination, on serum LDL and small dense LDL cholesterol levels in overweight and obese patients with hypercholesterolaemia. Curr. Med. Res. Opin. 2008;24(7):1919–1929. doi: 10.1185/03007990802177150. [DOI] [PubMed] [Google Scholar]
- Namıduru E.S., Tarakçoğlu M., Namıduru M., Kocabaş R., Erbağcı B., Meram I., Karaoğlan I., Yılmaz N., Cekmen M. Increased serum nitric oxide and malondialdehyde levels in patients with acute intestinal amebiasis. Asian Pac. J. Trop. Biomed. 2011;1(6):478–481. doi: 10.1016/S2221-1691(11)60104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niroumand S., Khajedaluee M., Khadem-Rezaiyan M., Abrishami M., Juya M., Khodaee G., Dadgarmoghaddam M. Atherogenic Index of Plasma (AIP): a marker of cardiovascular disease. Med. J. Islam. Repub. Iran. 2015;29:240. [PMC free article] [PubMed] [Google Scholar]
- Oliveira Godoy Ilha A., Sutti Nunes V., Silva Afonso M., Regina Nakandakare E., da Silva Ferreira G., de Paula Assis Bombo Renata, Rodrigues Giorgi R., Marcondes Machado R., Carlos Rocha Quintão E., Lottenberg A.M. Phytosterols supplementation reduces endothelin-1 plasma concentration in moderately hypercholesterolemic individuals independently of their cholesterol-lowering properties. Nutrients. 2020;12(5):1507. doi: 10.3390/nu12051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- Peterson D.W. Plant sterols and tissue cholesterol levels. Am. J. Clin. Nutr. 1958;6(6):644–649. doi: 10.1093/ajcn/6.6.644. [DOI] [PubMed] [Google Scholar]
- Piironen V., Lindsay D.G., Miettinen T.A., Toivo J., Lampi A. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000;80(7):939–966. [Google Scholar]
- Pollak O.J. Successful prevention of experimental hypercholesteremia and cholesterol atherosclerosis in the rabbit. Circulation. 1953;7(5):696–701. doi: 10.1161/01.cir.7.5.696. [DOI] [PubMed] [Google Scholar]
- Racette S.B., Lin X., Lefevre M., Spearie C.A., Most M.M., Ma L., Ostlund R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. Am. J. Clin. Nutr. 2010;91(1):32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D.R., Chawan C.B., Pulusani S.R. Influence of milk and thermophilus milk on plasma cholesterol levels and hepatic cholesterogenesis in rats. J. Food Sci. 1981;46(5):1339–1341. [Google Scholar]
- Ras R.T., Geleijnse J.M., Trautwein E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomized controlled studies. Br. J. Nutr. 2014;112(2):214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reeves P.G., Nielsen F.H., Fahey G.C., Jr. 1993. AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. [DOI] [PubMed] [Google Scholar]
- Ribas S.A., Sichieri R., Moreira A., Souza D.O., Cabral C., Gianinni D.T., Cunha D.B. Phytosterol-enriched milk lowers LDL-cholesterol levels in Brazilian children and adolescents: double-blind, cross-over trial. Nutr. Metabol. Cardiovasc. Dis. 2017;27(11):971–977. doi: 10.1016/j.numecd.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., Ahmed M., Aksut B., Alam T., Alam K. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Quispe C., Sharifi-Rad J., Cruz-Martins N., Nigam M., Mishra A.P., Konovalov D.A., Orobinskaya V., Abu-Reidah I.M., Zam W. Phytosterols: from preclinical evidence to potential clinical applications. Front. Pharmacol. 2021;11:1819. doi: 10.3389/fphar.2020.599959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sialvera T.E., Pounis G.D., Koutelidakis A.E., Richter D.J., Yfanti G., Kapsokefalou M., Goumas G., Chiotinis N., Diamantopoulos E., Zampelas A. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr. Metabol. Cardiovasc. Dis. 2012;22(10):843–848. doi: 10.1016/j.numecd.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Srisawasdi P., Chaloeysup S., Teerajetgul Y., Pocathikorn A., Sukasem C., Vanavanan S., Kroll M.H. Estimation of plasma small dense LDL cholesterol from classic lipid measures. Am. J. Clin. Pathol. 2011;136(1):20–29. doi: 10.1309/AJCPLHJBGG9L3ILS. [DOI] [PubMed] [Google Scholar]
- Stein E.A., Myers G.L. National cholesterol education program recommendations for triglyceride measurement: executive summary. The national cholesterol education program working group on lipoprotein measurement. Clin. Chem. 1995;41(10):1421–1426. [PubMed] [Google Scholar]
- Tamime A.Y., Robinson R.K. Elsevier; 2007. Tamime and Robinson's Yoghurt: Science and Technology. [Google Scholar]
- Trautwein E.A., Duchateau G.S., Lin Y., Mel'nikov S.M., Molhuizen H.O., Ntanios F.Y. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Technol. 2003;105(3-4):171–185. [Google Scholar]
- Tuzcu Z., Orhan C., Sahin N., Juturu V., Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1583098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos M., Moreno J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005;39(1):91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kumazaki S., Kusunoki K., Inoue T., Maeda Y., Usui S., Shinohata R., Ohtsuki T., Hirohata S., Kusachi S. A high-fat and high-cholesterol diet induces cardiac fibrosis, vascular endothelial, and left ventricular diastolic dysfunction in SHRSP5/Dmcr rats. J. Atherosclerosis Thromb. 2017 doi: 10.5551/jat.40956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2017. The Investment Case for Noncommunicable Disease Prevention and Control in the Kingdom of Saudi Arabia: Return on Investment Analysis & Institutional and Context Analysis. [Online] [Google Scholar]
- WHO . 2019. Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. http://who.int/gho/mortality_burden_disease/en/ [Google Scholar]
- Yahya M.A., Alhaj O.A., AL-Khalifah A.S., Almnaizel A.T. Hypocholesterolemic effect of camel milk on rats fed a high-cholesterol diet. Emir. J. Food Agric. 2018:288–294. [Google Scholar]
- Yuan C., Ding X., Jiang L., Ye W., Xu J., Qian L. Effects of dietary phytosterols supplementation on serum parameters, nutrient digestibility and digestive enzyme of white feather broilers. Ital. J. Anim. Sci. 2021;20(1):2102–2109. [Google Scholar]
- Zuberu J., Saleh M., Alhassan A.W., Adamu B.Y., Aliyu M., Iliya B.T. Hypolipidemic and antioxidant activity of camel milk on poloxamer-induced hyperlipidemia in rats. J. Afr. Assoc. Physiol. Sci. 2017;5(1):58–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.