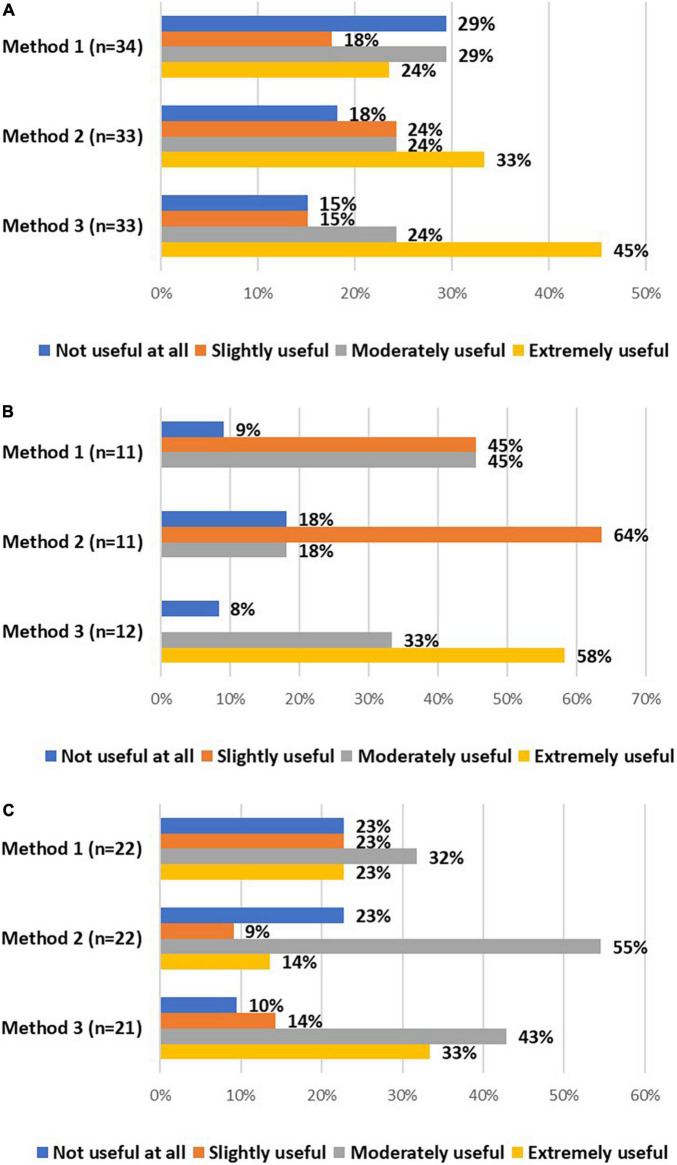
FIGURE 4.
The views of investigators (A), DPOs/legal experts (B), and EC members (C) on the usefulness of method 1 (i.e., if written consent by the research participant is not possible, IC could be given orally by the research participant in the presence of an impartial witness, who is required to sign and date the IC form), method 2 (i.e., the research participant and the person obtaining consent sign and date separate IC forms and an appropriately signed and dated IC should be obtained from the participant as soon as possible), and method 3 (i.e., using validated electronic systems) to obtain IC from COVID-19 patients.