Abstract
Objectives:
Zoonotic pathogens on dairy farms are a known risk for people who work and live there. Exposure and/or transmission of Salmonella serovars, E. coli (O157; H7), Campylobacter jejuni, and Cryptosporidium parvum have been documented to occur in the dairy farm environment. Social ecological factors have been identified as determinants of preventive behaviors of people at risk of infectious diseases.
Methods:
This study described the effect of socio-ecological factors on selected zoonotic bacterial and protozoal diseases in 42 workers of two dairy farms.
Results:
Occupational exposure to Salmonella ser. Dublin, E. coli, and Campylobacter spp. was confirmed. Self-efficacy and negative workplace perceptions were risk factors for Salmonella Dublin exposure (OR = 1.43[95% CI 1.11–2.22] & 1.22 [95% CI 1.02–1.53] respectively,). Additionally, safety knowledge and risk perceptions were protective factors of exposure (OR = 0.90 [95% CI 0.79–1.00]). Positive perceptions of supervisors and coworkers was a protective factor of Campylobacter exposure (OR = 0.89 [95% CI 0.79–0.98]).
Conclusion:
Results indicated that the presence of a supporting organizational environment, good communication with supervisors and coworkers, and training on prevention of zoonotic diseases would potentially reduce occupational exposures to zoonotic diseases on these farms.
Keywords: Socio-ecological factors, dairy, principal component analysis, risk, workers, zoonoses
Introduction
Dairy cattle operations represent a working environment with a high risk of exposure to zoonotic pathogens.1 People working or living on a farm, farm visitors, service providers, and veterinarians are the most at-risk for contracting zoonotic infections. Many pathogenic agents found on dairy farms are associated with diseases in farmers, farm workers, service providers, and consumers of dairy products.2 Among the most common pathogens found, Salmonella, E. coli (O157:H7), Campylobacter jejuni, and Cryptosporidium parvum are of particular interest due to their abundance in the farm environment and the severity of illness with which they have been associated.3–7
It has been demonstrated that the behavior of the at-risk person can affect their exposure to infectious agents.8–10 The prevention of zoonotic diseases in animal-human interfaces can be challenging, as it is affected by the complexity of the socio-ecological systems that drive preventive behavior.11 As demonstrated in other settings, the implementation of consistent and robust preventive measures can change the behavior of at-risk persons and successfully decrease exposure to risk factors.12–14
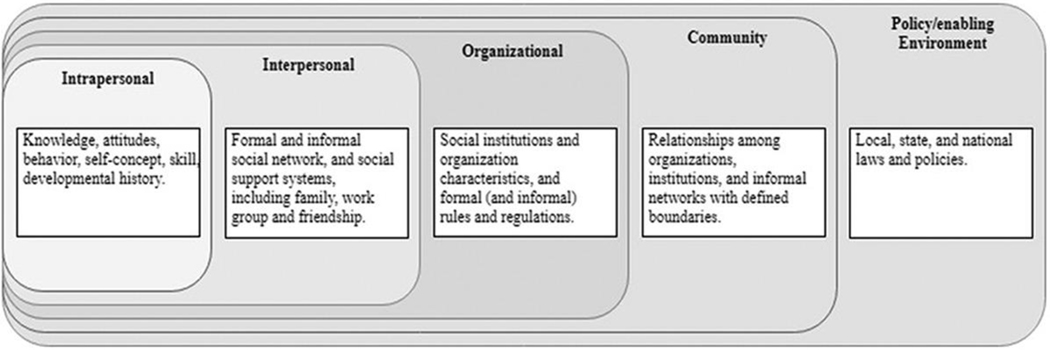
The Social Ecological Model (SEM) is a theory-based framework for understanding the complex interactive personal and environmental factors that affect preventive behaviors in specific settings. The SEM is a model composed of hierarchically organized levels that comprehensively provide all the potential factors that could affect the preventive behavior of a person. These levels are intrapersonal, interpersonal, organizational, community, and enabling level. The SEM framework has been used broadly in addressing health prevention, particularly in public health research.9 The SEM provides a useful framework for achieving a better understanding of the multiple factors that impact prevention on the farm. Therefore, it can be used to inform the development or improvement of comprehensive and compelling intervention strategies directly targeting mechanisms of behavior change at different levels of influence (Figure 1). Using the SEM framework, this research aimed to identify the potential social ecological factors that affect the exposure of dairy farmworkers to important zoonotic pathogens, specifically those associated with microbial infection.
Figure 1.
Basic SEM model. Adapted from McLeroy et al.13.
Materials and methods
A cross-sectional research design was used to simultaneously obtain socio-ecological data and microbiological samples as indicators of zoonotic pathogen exposure for dairy farmworkers.
Population access
A database of dairy farms was created using the publicly available list of the Colorado Livestock Association. Contacts were approached via e-mail, at least two times, and asked for access to their farms and to the workers for participation. If no e-mail response was obtained, phone contact was attempted. With farm access granted, and after receiving authorization from the IRB (The Colorado State University’s Institutional Review Board, Protocol ID 15–6168H), the potential participants (i.e, dairy workers) were presented with a short verbal introduction, a letter of invitation/consent, and a monetary incentive (USD $20 each). Based on the reported high-risk areas for exposure to zoonotic diseases, workers within milking parlors, calf rearing areas, and maternity and hospital areas were selected.
Collection of socio-ecological data
A questionnaire was developed and validated for collecting information regarding the SEM factors (available from the correspondent author upon request). The sources of measurement validity for this instrument were evaluated using the Standards for Educational and Psychological Testing 2014 edition.30 The content of the questionnaire was obtained using two parallel sources. A comprehensive literature review and a qualitative study in search of recommended prevention practices and previously validated items relevant to SEM factors.
The qualitative study was conducted by interviewing experts with demonstrated knowledge or experience on prevention of zoonotic diseases in dairy farms. Among them were field and university veterinarians, epidemiologists, public health, occupational health, and occupational psychology experts. Their responses were qualitatively analyzed using grounded theory analysis. This analysis yields a total of 16 themes, which were then located in the levels of a SEM.
Simultaneously, a scoping literature review was conducted with the objective of obtaining evidence of social ecological (SE) factors affecting the prevention of infectious diseases. Initially, 334 papers were title and abstract screened; 42 of those were wholly screened, and 19 were finally found to contain evidence of SE factors affecting the prevention of infectious diseases. With the content items identified, either a literature exploration was performed to find previously validated questions, or, if no question was available from previously reported studies, then a question or questions were drafted aimed at measuring that content item. For instance, “self-efficacy”. was identified by both studies as a factor that can affect preventive behavior of the people at risk. Several previously validated items (questions) measuring self-efficacy were included: e.g., “how confident are you of your abilities to perform the job tasks assigned?” In a similar way, “knowledge of zoonotic diseases” has been reported as a relevant factor. This factor was assessed by several questions measuring different topics of knowledge such as definitions, prevention practices, consequences, and basic biology and disease mechanisms.
Since the target population was largely Hispanic, a double-blinded translation was performed by two native Spanish speakers independently, and the differences were discussed by the two translators until consensus was achieved. The translated questionnaire was then tested on a small sample (n = 5) of Spanish speaking dairy workers. All these steps were used for refinement and adjustment of the final questionnaire.
Sample collection and laboratory procedures
Sample collection
Before the start of the work shift, three pieces of absorbent material (NON24265 Medline Northfield IL) were placed over the worker’s clothes. The potentially contaminated materials were collected after a period of 15 to 20 minutes, along with nitrile gloves and boots swabs (EZ-Dry-Pur, World Bioproducts, Woodinville WA). Concurrently, pooled samples of railings, handles, and control boards were colected in the areas of interest as a part of environmental sampling. All samples were immediately put into sterile Buffered Peptone Water (BPW). The samples were individually labeled and transported to the laboratory for further processing.
Isolation and confirmation of zoonotic pathogens
The homogenized BPW suspensions from the samples were divided into four equal reserved aliquots for testing for Salmonella, Campylobacter, Cryptosporidium, and E. coli O157:H7. The isolation and identification of Salmonella and Campylobacter were made through culture, isolation, and preliminary testing, followed by PCR confirmation. Briefly, positive serogroups were analyzed using a multiplex-PCR for confirmation of serovar Typhimurium and serovar Dublin.31,15,16 The PCR for the Campylobacter isolation was performed on agglutination positive samples for identification of the lpxA sequence, modified from the method of Klena et al.17 using the same cited primers sequences.
E. coli was identified by direct PCR according to Fode-Vaughan et. al.18 Merifluor® Giardia/Cryptosporidium detection kits (Meridian Bioscience, Cincinnati OH) were used for identification of Cryptosporidium oocysts in the samples. The preparation of the samples was performed following the manufacturer’s instructions.
Data analysis
Descriptive statistics and basic inferential statistics were used for characterizing the population of interest and to find out bivariate and multivariate associations between the observed variables. Confirmative factor analysis was used for the establishment of geometric co-variability (multivariate) relationships among the social-ecological variables. Principal components analysis (PCA) with varimax rotation was the chosen methodology. Parallel analysis was used to determine the number of factors to retain.19 Correlations (loadings) above |0.4| between factors and principal components were used as a cutoff point for interpreting the retained factors.
Variables and subjects with a large proportion of missing values were removed (>20% of cells with missing values). The rest of the missing values were imputed using an Iterative Principal Components Analysis method as described by Josse et. al.20 This process provides scores and loadings minimizing the least-squares criterion on the observed entries, which is optimal according to the PCA criterion. The retained factors were then used as independent variables to find bivariate associations with indicators of exposure. All statistical tests were run on R statistical software.21 The following are the R packages used: stats, missMDA, parallel, psych, arms, and Hmisc.
Results
Using e-mail and phone, 38 farms were contacted. This is approximately 30% of all the farms in Colorado (https://www.dairymax.org/). Of the farms, four agreed to participate and two declined; the remaining farms either never responded, or the contact information was inaccurate. One of the four farms ceased communications in the preparation phase, and one additional farm was located out of the reach of the area of influence and was dropped from the study. From the remaining two farms (5% of the farms reached), one was a conventional farm with approximately 1200 milking cows, and the other was an organic farm with approximately 8000 milking cows. Overall, 42 workers out of 52 that were reached (9 from one farm and 33 from the other farm) were sampled and interviewed.
Results
Laboratory results
Several samples from workers were positive for Salmonella, Campylobacter, and E. coli, but none of the samples were positive for Cryptosporidium (Table 1). The only Salmonella serotype identified by PCR was Salmonella Dublin. There were 11 environmental samples analyzed: 4 from milking parlors, 4 from hospital units, 2 from calf pens, and 1 from a maternity area. Of the samples, 5 were positive to Salmonella’s O antigen (A-I + Vi) (3 milking parlors and 2 from hospital areas); 3 of those (milking parlors) tested positive for serovar Dublin by PCR. Also, 5 samples (2 milking parlors, 2 hospital areas, 1 maternity) were positive for Campylobacter by latex agglutination; of those, one (milking parlor) was identified as C. jejuni by PCR.
Table 1.
Summary of the frecuencies and proportions of positive samples per laboratory tests.
| Chest piece | Boots | Gloves/sleeves | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Test | Frequency | Proportion | Frequency | Proportion | Frequency | Proportion | Frequency | Proportion |
| O ant. Salm. (A-I + Vi)* | 14/42 | 0.33 | 7/42 | 0.17 | 13/42 | 0.31 | 34/126 | 0.27 |
| PCR Salm. InvA | 4/14 | 0.29 | 3/7 | 0.43 | 4/13 | 0.31 | 11/34 | 0.32 |
| PCR S. Typhimurium. | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| PCR S. Dub. | 5/14 | 0.36 | 4/7 | 0.57 | 6/13 | 0.46 | 15/34 | 0.44 |
| L. aglutination. Campy.* | 8/42 | 0.19 | 4/42 | 0.10 | 15/42 | 0.36 | 27/126 | 0.21 |
| PCR C. jejuni | 2/8 | 0.25 | 2/4 | 0.50 | 2/15 | 0.13 | 6/27 | 0.22 |
| PCR C. coli | 1/8 | 0.13 | 1/4 | 0.25 | 0 | 0.00 | 0 | 0.00 |
| PCR E. coli† | 1/30 | 0.03 | 1/30 | 0.03 | 4/30 | 0.13 | 5/90 | 0.06 |
| Cryptosporidium DFA | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
PCR proportion of positive samples for Salmonella and Campylobacter are of those preliminarily identified with serotyping and aglutination tests.
Only.
Population demographics
Of the population surveyed, 53% (22/41) had only primary education, and 34% (14/42) had been enrolled up to middle school. Only 12% (5/41) of interviewees received a high school education or greater. Of the population, 63% were men (26/42), and 50% were younger than 30 years-of-age (21/42, range 21 to 51 years-old). Out of 42 workers, 37 (88.1%) were immigrants from Hispanic countries including Mexico, Guatemala, Honduras, and Colombia.
Knowledge and training items
The questionnaire included several questions aimed at measuring the knowledge of prevention of zoonotic diseases and safety training. Following are some of the relevant findings.
Of the respondents, 505 (21/42) scored over 70 (out of 100) points on the knowledge score; while, 90% of respondents were either not sure or did not know what the recommended vaccines for people working on dairy farms were. There were 49% (20/41) of respondents who indicated that people that do not work on farms are not exposed to zoonotic diseases, and 51% (21/41) indicated that pathogens cannot be carried home by workers. Similarly, 34% (14/41) were unsure whether zoonotic diseases can harm workers permanently.
Regarding training, the most recalled training topic was “steps to follow in case of a safety event” (62%, 26/42), followed by proper “personal protective equipment (PPE) use” (60%, 25/42), injuries and accident prevention (52%, 22/42), then hygiene practices, sources of safety information, and handwashing (24%−31%, 10–13/42). Zoonotic diseases and sanitation practices were the least recalled content topic (5%−14%, 2–6/42). Respondents reported that the most influential persons regarding safety practices were their supervisor (40%, 17/42) and coworkers (26%, 11/42).
Prevention practices and risk attitudes and perceptions
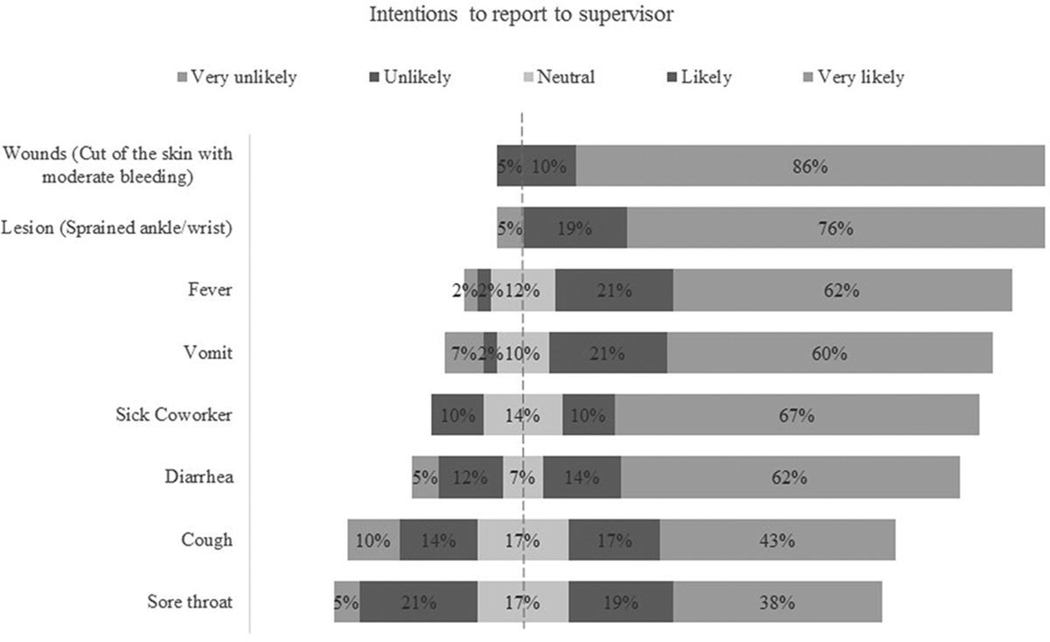
Participants showed a high rate of intention to report injury-related events. In contrast, they were less likely to report signs of intestinal infections (vomiting, diarrhea, or fever), and even less likely to report signs of respiratory illness (Figure 2). Similarly, about 90% of respondents “very likely” intended to seek medical care in case of a wound, and 72% of respondents indicated “likely” and “very likely” intentions to seek medical care in case of gastrointestinal infection symptoms (Figure 2).
Figure 2.
Percentages distribution of the intentions of reporting to supervisor in case of a health event (n = 42).
Regarding animal exposure, 33% of 42 respondents (79%) thought being exposed to a sick animal implied at least a moderate level of hazard. About half of the participants (49%, 20/41) were “a little” or “not afraid at all” of getting a disease at work; however, 64% (26/42) were at least “moderately” concerned that they could carry diseases to their families. A concerning finding was that 30% (12/41) of participants disagreed or strongly disagreed that the use of PPE reduces the risk of diseases’ exposure, and 25% strongly disagreed that washing hands reduces the risk of disease transmission.
Multivariate analysis
Confirmatory factor analysis
Based on the parallel analysis, 12 components (factors) were retained, and they contributed to 81% of the total variance among all factors. The first two factors were “Self-efficacy” and “Knowledge and Risk perception,” and they contributed with 11% and 10% to the maximum variance, respectively. “Attitude toward infectious disease-related symptoms” (factor 6) contributed 9% of the total variance. The detailed interpretation of all factors is summarized in Table 2.
Table 2.
Interpretation of retained factors by the principal component analysis. In parenthesis () is the number of variables that had loadings >|0.4|.
| Factors | Proportion of variance explained | Underlying variable |
|---|---|---|
| Factor 1(11) | 11% | Self-efficacy |
| Workability | ||
| Job-related self-efficacy | ||
| Preventive practices ability | ||
| Other | ||
| Interest in infectious diseases | ||
| Communication with top management | ||
| Trust in management | ||
| Factor 2(11) | 10% | Knowledge & risk perception |
| Knowledge relevant to zoonotic diseases | ||
| Risk perception and concerns | ||
| Other | ||
| Seeking health care or reporting infectious disease symptoms | ||
| Supervisor safety knowledge perception | ||
| Factor 3(9) | 9% | Injuries attitudes |
| Reporting | ||
| Seeking healthcare | ||
| Other health problems attitudes | ||
| Reporting vomiting | ||
| Reporting diarrhea | ||
| Other | ||
| Job satisfaction | ||
| Use of face/eye protection | ||
| Training trust | ||
| Factor 4(12) | 9% | Management attitudes and perceptions |
| Organizational commitment | ||
| Trust in management | ||
| Coworkers support | ||
| Other | ||
| Discrimination perception (inverse) | ||
| Factor 5(11) | 9% | Perceptions and attitudes of supervisors and coworkers |
| Reporting health problems to supervisor | ||
| Trust in supervisor | ||
| Supervisor communication | ||
| Trust in coworkers | ||
| Other | ||
| Use of Sleeves | ||
| Use of Apron | ||
| Factor 6(9) | 9% | Infectious diseases related symptoms attitudes |
| Seeking healthcare attitudes | ||
| Other | ||
| Workability | ||
| Factor 7(7) | 9% | Workplace Satisfaction |
| Job control | ||
| Job satisfaction | ||
| Trust in management | ||
| Reporting retaliation (lack of) | ||
| Other | ||
| Perception of coworkers’ safety attitudes | ||
| Training trust | ||
| Factor 8(7) | 8% | Workplace perceptions of supervisors and coworkers |
| Supervisor and coworker’s communication quality | ||
| Supervisor trust | ||
| Role ambiguity (lack of) | ||
| Provided safety resources perception | ||
| Other | ||
| Educational level | ||
| Factor 9(5) | 9% | Perceptions and reporting of personal protective equipment use |
| Factor 10(6) | 6% | Training perception |
| Language of training | ||
| Negative perception of training sessions | ||
| Other | ||
| Role conflict | ||
| Poor communication with managers | ||
| Organizational trust | ||
| Experience in dairy farms | ||
| Factor 11(7) | 6% | Workplace negative Perceptions |
| Role overload | ||
| Job control (lack of) | ||
| Coworkers’ support (safety concern) | ||
| Other | ||
| Cultural relatability | ||
| Training frequency | ||
| Reporting health problems to supervisor | ||
| Using work boots | ||
| Factor 12(5) | Training Miscellaneous | |
| Training frequency | ||
| Use of examples/demonstrations | ||
| Being involved in training demonstrations | ||
| Adequate safety information | ||
| Other | ||
| Workability | ||
| Organizational commitment |
Associations of factors and laboratory results
Factor 1 was found to be a risk factor for exposure to zoonotic microorganisms (OR 1.11, 95% CI 1.02–1.25); however, Factor 5 was found to be protective (OR 0.9, 95% CI 0.81–0.99). When analyzing the pathogens separately, Factors 1 and 11 were identified as risk factors for S. enterica. ser. Dublin exposure (OR 1.43, 95% CI 1.11–2.22; OR 1.22, 95% CI 1.02–1.52, respectively), and Factor 2 was identified as protective (OR 0.91, 95% CI 0.81–0.99). Factor 5 was found as a protective factor for Campylobacter spp. (OR 0.89, 95% CI 0.68–0.97). All other results are summarized in Table 3
Table 3.
Significant (p-value≤0.05) and relevant (0.05 < p-value≤0.1) bivariate logistic regression analysis results of principal component factors and laboratory results
| Exposure to either pathogen | PCR Salm Dublin | Lat Ag. Campy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 2.5% | 97.5% | p-value | OR | 2.5% | 97.5% | p-value | OR | 2.5% | 97.5% | p-value | |
| Factor 1 | 1.11 | 1.02 | 1.25 | 0.04 | 1.43 | 1.11 | 2.22 | 0.04 | 1.09 | 1.00 | 1.21 | 0.09 |
| Factor 2 | 0.93 | 0.85 | 1.00 | 0.08 | 0.91 | 0.82 | 0.99 | 0.05 | 0.92 | 0.84 | 1.00 | 0.07 |
| Factor 3 | 0.84 | 0.67 | 0.99 | 0.08 | 0.84 | 0.68 | 0.97 | 0.07 | ||||
| Factor 4 | 0.90 | 0.79 | 1.00 | 0.06 | ||||||||
| Factor 5 | 0.90 | 0.81 | 0.99 | 0.04 | 0.89 | 0.79 | 0.98 | 0.03 | ||||
| Factor 9 | 0.90 | 0.79 | 1.00 | 0.06 | 0.89 | 0.78 | 1.00 | 0.07 | ||||
| Factor 11 | 1.22 | 1.02 | 1.53 | 0.04 | ||||||||
Discussion
Campylobacter and Salmonella have been isolated in dairy farms22 and are potentially dangerous agents. To the authors’ knowledge, this is the first report of confirmation of contamination of worker’s gloves or work clothes with these pathogens. Stenkamp-Strahm et al.4 reported gloves contaminated with E. coli O15:H7. We found a few samples containing E. coli O15:H7; however, these results were not included in the final factor analysis, since not all samples were processed (30/42), and there was a significant amount of missing data from the survey on the subjects with positive results. We could not detect any sample containing Cryptosporidium; this is perhaps due to the low probability of identifying this protozoan with the technique used. The limit detection of this technique is 103 oocyst per gram of sample,23 which is more suitable for identification of the parasites directly on fecal samples. Other concentration techniques were considered during the planning phase of the project; however, they were not included based on laboratory capabilities, logistic barriers, or low expected exposure rates. For the same reasons, other pathogens of relevance were not included in this study.
Salmonella and Campylobacter occurred concomitantly in our samples. The identification of these two agents in the same samples has been described previously, predominantly within contaminated food (avian food products), which may indicate a common source of contamination.1,24 In this case, finding both microorganisms on the same workers may indicate that exposure to these agents could be driven by shared environmental factors in these workers including the common cattle source. S. enterica. ser. Dublin serotype has been found on dairy farms before and has been associated with large dairy farms.5
As per our observations, we can conclude that either the farms are not including zoonotic disease prevention in their preventive programs or the current delivery methods are not effective, as the information is not properly recalled by the workers. An unexpected result was that there was no association between training variables and knowledge, indicating that the knowledge measured could have been acquired from other sources. Regarding the methods of knowledge delivery, experts recommend the use of different training methods and participatory activities to increase the effectiveness of knowledge transfer.12
Injury prevention training was more evident than disease prevention training. This was reflected in the workers’ perceptions toward zoonotic disease risks, where respondents’ likelihoods to report injuries were higher than infectious diseases. We interpreted 12 components (Factors) from the PCA (Table 2). Each retained component compiled between 7 and 12 variables. The interpretation of these Factors was based on the hypothesized SEM model.
“Self-efficacy” (Factor 1) and “workplace negative perceptions” (Factor 11) were risk factors of exposure to S. enterica. ser. Dublin. In contrast, “knowledge & risk perception” (Factor 2) was a protective factor. Self-efficacy related overconfidence has been reported as a risk factor for injuries on farms.25 According to Neal and Griffin,26 “Overconfidence is a strong source of bias in evaluating risk and has been related to unsafe behavior.” In a study with Latinx roofers, Hung et al.27 reported that overconfidence leads to disvalue work safety through their perception that they know “a lot” about safety. It is plausible that the phenomena described are similar among dairy workers. However, Conchie et al.28 stated that “trust (as a self-efficacy driver) and its role in shaping organizational safety is poorly understood”. They reported that some of the analyzed papers found positive associations between trust and safety climate, and negative associations between trust and safety performance, which may seem contradictory. This may indicate that there are other underlying complex factors and interactions that affect differently the relationship between self-efficacy, safety performance and work performance. The authors recommend further research to elucidate the complexity of the relationships between these constructs.
Safety knowledge has been related to safety performance. However, there is always a condition that precedes this relationship, that is, a robust and supportive organizational safety climate.29 This supports our findings and reinforces the findings that “knowledge of zoonotic diseases and risk perceptions” might be a protective factor against zoonotic exposure that could be further clarified in subsequent research.
“Perceptions and attitudes of supervisors and coworkers” (Factor 5) was found to be protective for Campylobacter exposure. This observation supports the role of supervisors and coworkers as drivers of safety performance. It has been described that supervisors have a significant influence on the practice of prevention at work.26 All of this must be framed under excellent job relationships and communication norms and a supportive organizational environment. The difference in detecting social ecological factors related to one but not the other pathogen may be due to the differences in organizational factors. Further research is needed to broaden our understanding of the effect of organizational factors on exposure to zoonotic agents.
The response rate of workers within the accessed farms was high (78%), and an economic incentive, in addition to a history of collaboration between the farms and the university, played a key role in this outcome. However, despite our efforts, farm recruitment was significantly challenging. There is a noticeable gap between scientific understanding and producers’ perceptions; hence, we believe that efforts to improve the collaboration with farms is necessary to address the issue of exposure to zoonotic pathogens.
Conclusions
We found evidence that “Knowledge and risk perception” are protective factors of exposure to zoonotic diseases. Based on this, the frequent inclusion of infectious zoonotic diseases’ prevention topics on the farm’s safety information pathways may well reduce the risk of zoonotic disease exposure. Identification of early signs of infection, when to seek health care, recommended vaccines, causes and mechanisms of disease transmission, and potentially serious consequences of diseases should be frequent content topics in workers’ safety training. Additionally, it is worth considering some effective and culturally congruent training methods. Interactive, participatory, and demonstrative transfer methods increase the effectivity of knowledge acquisition.
Our study supports the role of supervisors and coworkers as effective channels of safety information. According to this, ensuring that supervisors and experienced coworkers provide accurate and precise safety information may help reduce exposure to zoonotic diseases. A supportive work environment can increase the efficacy of this channel.
We found evidence that self-efficacy and negative workplace perceptions are risk factors of exposure to zoonotic diseases. Thus, the inclusion of awareness of overconfidence on safety training programs and maintaining good communication and work environment among all workers may decrease the risk of exposure in these farms.
Confirmed by our laboratory results, we verified the exposure of dairy workers to potentially dangerous zoonotic pathogens. We confirmed that these pathogens could indeed be splashed as high as the chest area and this may indicate that exposure to facial areas is plausible. Use of face and nose/mouth protection should be instructed and encouraged.
We observed that, even though not significant at the 0.05 level, other factors are associated with the pathogens detected (Table 3). We hypothesize that these factors may influence the occupational exposure to zoonotic diseases and encourage further scientific efforts to test such. Due to the limited number of participant farms, the results found cannot be extrapolated. Despite our efforts, our farm sample was low, and the access to more workers was limited. In the analysis above and according to the literature, organizational factors are relevant drivers of preventive behavior; however, with just two farms, drawing more robust conclusions is constrained. However, the findings described herein can be used as a starting point to further explore whether these factors hold true as determinants of zoonotic exposure in other settings.
Acknowledgments
This study was supported in part with funds from the High Plains Intermountain Center for Agricultural Health and Safety (HICAHS) Pilot/Feasibility Research Projects grants program CDC/NIOSH with grant number U54.OH0008085. Special thanks to the farm managers, supervisors, and workers who collaborated in this study. The study would not have been possible without the contribution from the co-researchers, experts, and advisers who kindly shared their expertise, experience, knowledge, and wisdom on the development, implementation, and interpretation of this research study.
Additional information
Funding
This work was partially supported by Centers for Disease Control and Prevention - National Institute for Occupational Safety and Health (CDC-NIOSH) through the High Plains Intermountain Center for Agricultural Health & Safety (HICAHS) pilot projects grant (5 U54OH008085-16-00).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Salman M, Steneroden K. Important public health zoonoses through cattle. In: Sing A, ed. Zoonoses - Infections Affecting Humans and Animals. 1st ed. Springer, Dordrecht. 2014. p. 3–22. [Google Scholar]
- 2.Van Kessel JS, Santin-Duran M, Karns JS, Schukken Y. 21 - Tracing zoonotic pathogens in dairy production. In: Brul S, Fratamico PM, McMeekin TA, eds. Tracing Pathogens in the Food Chain. Cambridge (UK): Woodhead Publishing; 2011:503–526. [Google Scholar]
- 3.Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. Evidence supporting zoonotic transmission of cryptosporidium spp. in wisconsin. J Clin Microbiol. 2006;44(12):4303–4308. doi: 10.1128/JCM.01067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenkamp-Strahm C, McConnel C, Hyatt DR, Magnuson R, Tenneson P, Linke L. Prevalence of Escherichia coli O157 shedding in preweaned calves on Colorado dairies. J Food Prot. 2017;80(6):990–993. doi: 10.4315/0362-028X.JFP-16-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huston CL, Wittum TE, Love BC, Keen JE. Prevalence of fecal shedding of Salmonella spp in dairy herds. J Am Vet Med Assoc. 2002;220(5):645–649. doi: 10.2460/javma.2002.220.issue-5. [DOI] [PubMed] [Google Scholar]
- 6.Cummings K. The Epidemiology And Public Health Implications Of Salmonellosis In Dairy Cattle [Dissertation]. Ithaca (NY): Cornell Univesity; 2010. [Google Scholar]
- 7.Wesley IV, Wells SJ, Harmon KM, et al. Fecal shedding of Campylobacter and arcobacter spp. in dairy cattle. Appl Environ Microbiol. 2000;66(5):1994–2000. doi: 10.1128/AEM.66.5.1994-2000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albarracín D, Gillette JC, Earl AN, Glasman LR, Durantini MR, Ho M-H. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull. 2005;131(6):856–897. doi: 10.1037/0033-2909.131.6.856., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stice E, Shaw H, Nathan C. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132(5):667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panter-Brick C, Clarke SE, Lomas H, Pinder M, Lindsay SW. Culturally compelling strategies for behaviour change: A social ecology model and case study in malaria prevention. Soc Sci Med. 2006;62(11):2810–2825. doi: 10.1016/j.socscimed.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Donham KJ. Zoonotic diseases: overview of occupational hazards in agriculture. In: Agricultural Medicine: Rural Occupational and Environmental Health, Safety, and Prevention. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2006;44(1):39–42. [Google Scholar]
- 12.Menger LM, Rosecrance J, Stallones L, Roman-Muniz IN. A guide to the design of occupational safety and health training for immigrant, latino/a dairy workers. Front Public Health. 2016;4 (282):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 14.Gallant M, Maticka-Tyndale E. School-based HIV prevention programmes for African youth. Soc Sci Med. 2004;58(7):1337–1351. doi: 10.1016/S0277-9536(03)00331-9. [DOI] [PubMed] [Google Scholar]
- 15.Tennant SM, Diallo S, Levy H, et al. Identification by PCR of non-typhoidal salmonella enterica serovars associated with invasive infections among febrile patients in mali. PLoS Negl Trop Dis. 2010;4(3):e621. doi: 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malorny B, Hoorfar J, Bunge C, Helmuth R. Multicenter validation of the analytical accuracy of salmonella PCR: towards an international standard. Appl Environ Microbiol. 2003;69(1):290–296. doi: 10.1128/AEM.69.1.290-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klena JD, Parker CT, Knibb K, et al. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA. J Clin Microbiol. 2004;42(12):5549–5557. doi: 10.1128/JCM.42.12.5549-5557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fode-Vaughan KA, Maki JS, Benson JA, Collins MLP. Direct PCR detection of Escherichia coli O157: H7. Lett Appl Microbiol. 2003;37(3):239–243. doi: 10.1046/j.1472-765X.2003.01386.x. [DOI] [PubMed] [Google Scholar]
- 19.Franklin SB, Gibson DJ, Robertson PA, Pohlmann JT, Fralish JS. Parallel analysis: a method for determining significant principal components. J Veg Sci. 1995;6(1):99–106. doi: 10.2307/3236261. [DOI] [Google Scholar]
- 20.Josse J, Chavent M, Liquet B, Husson F. Handling missing values with regularized iterative multiple correspondence analysis. J Classif. 2012;29(1):91–116. doi: 10.1007/s00357-012-9097-0. [DOI] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 22.Kwan PSL, Birtles A, Bolton FJ, et al. Longitudinal study of the molecular epidemiology of Campylobacter jejuni in cattle on dairy farms. Appl Environ Microbiol. 2008;74(12):3626–3633. doi: 10.1128/AEM.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol. 1991;29(7):1323. doi: 10.1128/JCM.29.7.1323-1327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altekruse SF, Swerdlow DL, Stern NJ. Campylobacter Jejuni. Vet Clin North Am Food Anim Pract. 1998;14(1):31–40. doi: 10.1016/S0749-0720(15)30277-2. [DOI] [PubMed] [Google Scholar]
- 25.Pickett W, Brison RJ, Niezgoda H, Chipman ML. Nonfatal farm injuries in Ontario: A population-based survey. Accid Anal Prev. 1995;27(4):425–433. doi: 10.1016/0001-4575(94)00080-6. [DOI] [PubMed] [Google Scholar]
- 26.Neal A, Griffin MA. Safety climate and safety at work. In: Barling J, Frone MR, eds. The Psychology of Workplace Safety. Washington: American Psychological Association; 2004:15–34. [Google Scholar]
- 27.Hung Y-H, Winchester WW, Smith-Jackson TL, Kleiner BM, Babski-Reeves KL, Mills TH. Identifying fall-protection training needs for residential roofing subcontractors. Appl Ergon. 2013;44(3):372–380. doi: 10.1016/j.apergo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Conchie SM, Donald IJ, Taylor PJ. Trust: missing Piece(s) in the safety puzzle. Risk Anal. 2006;26(5):1097–1104. doi: 10.1111/j.1539-6924.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith‐Crowe K, Burke MJ, Landis RS. Organizational climate as a moderator of safety knowledge–safety performance relationships. J Organ Behav. 2003;24(7):861–876. doi: 10.1002/job.217. [DOI] [Google Scholar]
- 30.American Educational Research Association, American Psychological Association, National Council on Measurement in Education, Joint Committee on Standards for Educational and Psychological Testing (U.S.), eds. Standards for Educational and Psychological Testing. Washington, DC: American Educational Research Association; 2014. [Google Scholar]
- 31.Rahn K, De Grandis SA, Clarke RC, et al. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes. 1992;6(4):271–279. doi: 10.1016/0890-8508(92)90002-F. [DOI] [PubMed] [Google Scholar]