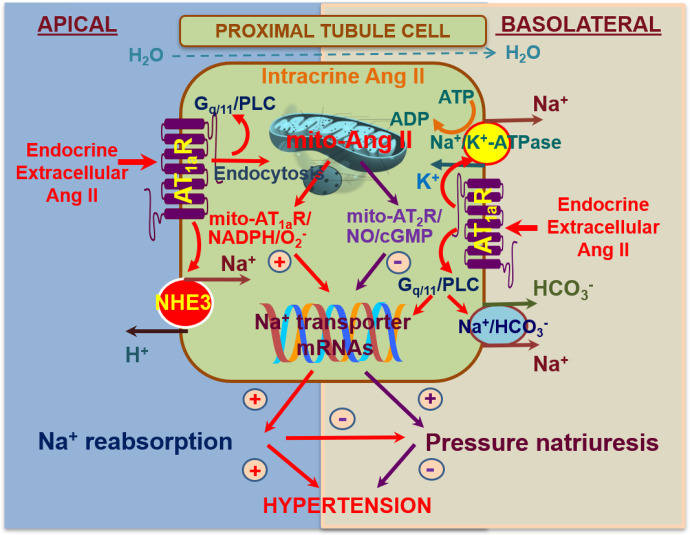
Fig. 7.
Scheme showing that extracellular (endocrine and paracrine) angiotensin (Ang) II is taken up via Ang II type 1a receptor (AT1aR)-mediated internalization and then results in Gq/11/phospholipase C (PLC) signaling. Under physiologic conditions (low Ang II), internalized Ang II is sorted to the lysosomal pathway for degradation, whereas AT1a receptors recycle back to the membrane. Alternatively, particular at sustained high extracellular Ang II levels, the Ang II–AT1aR complex may bypass the lysosomal degradation pathway, allowing its transport to mitochondria and nucleus, where Ang II activates AT1aR and/or Ang II type 2 receptor (AT2R) to alter mitochondrial oxidative and glycolysis stress responses. This may in turn alter the expression or activity of Na+/H+ exchanger 3 (NHE3) on the apical membranes, or Na+/K+-ATPase and the Na+/HCO3− cotransporter on the basolateral membranes in the proximal tubules. Thus, activation of the mitochondrial Ang II/AT1aR/O2− signaling will stimulate proximal tubule sodium reabsorption, impair the pressure-natriuresis response, and elevate blood pressure. Conversely, activation of the mitochondrial Ang II/AT2R/NO/cGMP signaling by overexpressing AT2R in the mitochondria will likely inhibit proximal tubule sodium reabsorption, augment the pressure-natriuresis response, and lower blood pressure. Modified from Li et al. (2020, 2021).