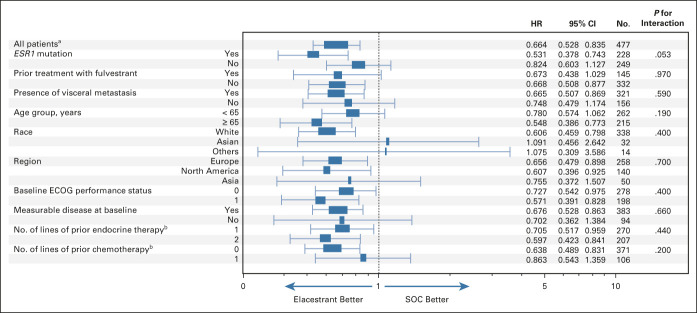
FIG 2.
Subgroup analysis of PFS in all patients. PFS, as assessed by blinded independent central review, in clinically relevant subgroups of patients with ER-positive/HER2-negative advanced breast cancer. Interaction P values were all nonsignificant indicating that elacestrant benefit on PFS is independent of subgroup. aNonstratified analysis. bIn the advanced setting. ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PFS, progression-free survival; SOC, standard of care.