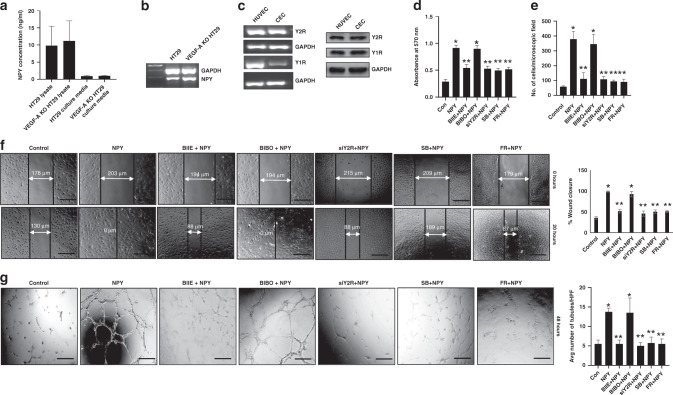
Fig. 5. NPY stimulates the angiogenic potential of colonic endothelial cells via Y2R expressed on these cells.
a ELISA assay, and b RT-PCR analysis, showed that HT29 and VEGF-A KO HT29 colon cancer cells synthesise and secrete significant amounts of NPY. c RT-PCR and western blot analysis indicated the presence of Y2 (Y2R) and Y1 (Y1R) NPY receptors in colonic endothelial cells (CEC) and human umbilical vein endothelial cells (HUVEC). NPY (100 nM) promoted proliferation (d MTT assay), mobilization (e in vitro transwell migration assay), migration (f scratch wound assay) and tubule formation (g in vitro tubulogenesis assay) of CEC. Blocking Y2R with BIIE0246 (1 µM) or silencing Y2R (siY2R) in CEC abrogated the effects of NPY. However, the Y1R antagonist, BIBO3304 (1 µM), had no effect on the actions of NPY. Furthermore, pretreatment of CEC with either SB203580 hydrochloride or FR180204 inhibited NPY-induced CEC proliferation, migration and tubule formation. Results are expressed as mean ± SEM; *P < 0.05 when compared to control cells and **P < 0.05 when compared to NPY-treated cells. Error bars represent SEMs from three independent experiments. Scale bar, 100 μm.