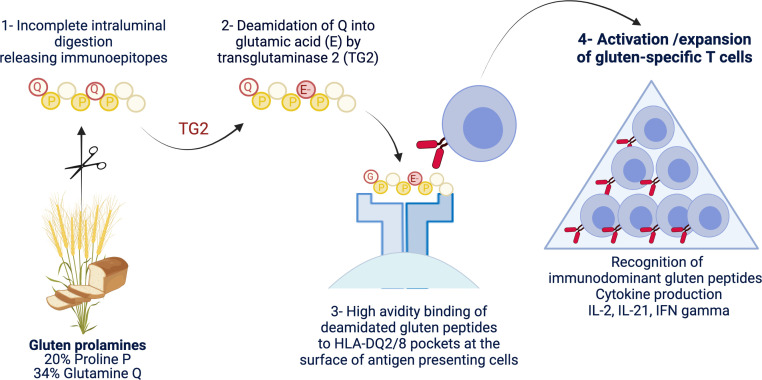
Figure 1.
Schematic representation of the driver antigluten response. The richness of gluten proteins in proline and glutamine underlies their ‘toxicity’ for patients with coeliac disease. Due to the lack of luminal endo-prolylpeptidases, proline-rich gluten proteins are incompletely digested and release large immunogenic peptides which can be modified by transglutaminase 2 (TG2). This enzyme selectively targets a subset of proline-rich and glutamine-rich sequences in gluten peptides and deamidate neutral glutamine residues (Q) into negatively charged glutamic acid (E). Gluten peptides harbouring negative charges on specific positions can bind with high avidity the peptide pockets of HLA-DQ2 or HLA-DQ8 molecules at the surface of antigen-presenting cells. The latter cells can then efficiently stimulate activation and expansion of gluten-specific CD4+ T cells that produce proinflammatory cytokines such as interleukin (IL)-2, interferon gamma (IFNγ) and IL-21. Created with Biorender.com.