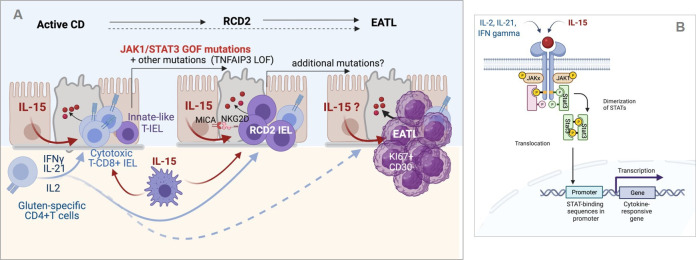
Figure 4.
Inflammation-driven lymphomagenesis in coeliac disease (CD). (A) Left panel: in active CD, cytokines produced by gluten-specific CD4+ T cells cooperate with interleukin (IL)-15 to stimulate the expansion and cytotoxic activation of polyclonal CD8+ T intraepithelial lymphocytes (IEL) that drive a cytolytic attack of epithelium. Middle panel: in type 2 refractory CD (RCD2), innate-like T IEL that have acquired somatic mutations and notably gain-of-function (GOF) mutations in Janus kinase 1 (JAK1) or signal transducer and activator of transcription 3 (STAT3) can clonally expand and outcompete CD8+ T IEL in the cytokine-rich CD intestine. Transformation is fostered by the acquisition of additional mutations, notably in TNFAIP/A20, which enhances the nuclear factor kappa B pathway, and in epigenetic regulators. Due to their natural killer (NK)-like functions, RCD2 IEL can induce severe epithelial lesions. Right panel: further accumulation of mutations in the clonally expanded RCD2 IEL ultimately lead to enteropathy-associated T cell lymphoma (EATL). While EATL generally develops from RCD2, it can also develop without a prior step of RCD2. (B) Schematic representation of the JAK1-STAT3 pathway. Its activation both by cytokines produced by CD4+ T cells and IL-15 explains its importance in the activation of normal CD8+ T IEL and RCD2 IEL. Created with Biorender.com.